
Teicoplanin is an semisynthetic glycopeptide antibiotic with a spectrum of activity similar to vancomycin. Its mechanism of action is to inhibit bacterial cell wall peptidoglycan synthesis. It is used in the prophylaxis and treatment of serious infections caused by Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus and Enterococcus faecalis.
The enzyme acyl-CoA hydrolase (EC 3.1.2.20) catalyzes the reaction
The enzyme ADP-dependent short-chain-acyl-CoA hydrolase (EC 3.1.2.18) catalyzes the reaction
The enzyme choloyl-CoA hydrolase (EC 3.1.2.27) catalyzes the reaction
The enzyme oleoyl-[acyl-carrier-protein] hydrolase (EC 3.1.2.14) catalyzes the reaction
Palmitoyl-CoA hydrolase (EC 3.1.2.2) is an enzyme in the family of hydrolases that specifically acts on thioester bonds. It catalyzes the hydrolysis of long chain fatty acyl thioesters of acyl carrier protein or coenzyme A to form free fatty acid and the corresponding thiol:
In enzymology, a [acyl-carrier-protein] S-malonyltransferase is an enzyme that catalyzes the chemical reaction

Acyl-coenzyme A thioesterase 8 is an enzyme that in humans is encoded by the ACOT8 gene.

Acyl-CoA thioesterase 2, also known as ACOT2, is an enzyme which in humans is encoded by the ACOT2 gene.

Cytosolic acyl coenzyme A thioester hydrolase is an enzyme that in humans is encoded by the ACOT7 gene.

Acyl-coenzyme A thioesterase 4 is an enzyme that in humans is encoded by the ACOT4 gene.

Acyl-coenzyme A thioesterase 11 also known as StAR-related lipid transfer protein 14 (STARD14) is an enzyme that in humans is encoded by the ACOT11 gene. This gene encodes a protein with acyl-CoA thioesterase activity towards medium (C12) and long-chain (C18) fatty acyl-CoA substrates which relies on its StAR-related lipid transfer domain. Expression of a similar murine protein in brown adipose tissue is induced by cold exposure and repressed by warmth. Expression of the mouse protein has been associated with obesity, with higher expression found in obesity-resistant mice compared with obesity-prone mice. Alternative splicing results in two transcript variants encoding different isoforms.

Acyl-coenzyme A thioesterase 12 or StAR-related lipid transfer protein 15 (STARD15) is an enzyme that in humans is encoded by the ACOT12 gene. The protein contains a StAR-related lipid transfer domain.

Acyl-CoA thioesterase 6 is a protein that in humans is encoded by the ACOT6 gene. The protein, also known as C14orf42, is an enzyme with thioesterase activity.

Acyl-CoA thioesterase 9 is a protein that is encoded by the human ACOT9 gene. It is a member of the acyl-CoA thioesterase superfamily, which is a group of enzymes that hydrolyze Coenzyme A esters. There is no known function, however it has been shown to act as a long-chain thioesterase at low concentrations, and a short-chain thioesterase at high concentrations.

Acyl-CoA thioesterase 13 is a protein that in humans is encoded by the ACOT13 gene. This gene encodes a member of the thioesterase superfamily. In humans, the protein co-localizes with microtubules and is essential for sustained cell proliferation.

Acyl-CoA thioesterase 1 is a protein that in humans is encoded by the ACOT1 gene.
Streptomyces parvulus is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces parvulus produces the peptide antibiotic Actinomycin D and the angiogenesis inhibitor borrelidin and manumycin A, himalomycin A, himalomycin B and kynurenine.
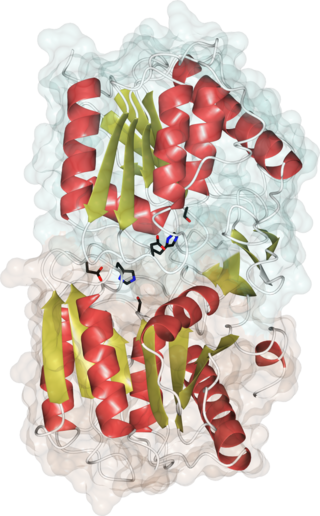
Acyl-protein thioesterases are enzymes that cleave off lipid modifications on proteins, located on the sulfur atom of cysteine residues linked via a thioester bond. Acyl-protein thioesterases are part of the α/β hydrolase superfamily of proteins and have a conserved catalytic triad. For that reason, acyl-protein thioesterases are also able to hydrolyze oxygen-linked ester bonds.

Borrelidin is an 18-membered polyketide macrolide derived from several Streptomyces species. First discovered in 1949 from Streptomyces rochei, Borrelidin shows antibacterial activity by acting as an inhibitor of threonyl-tRNA synthetase and features a nitrile moiety, a unique functionality in natural products., Borrelidin also exhibits potent angiogenesis inhibition, which was shown in a rat aorta matrix model. Other studies have been performed to show that low concentrations of borrelidin can suppress growth and induce apoptosis in malignant acute lymphoblastic leukemia cells. Borredlidin's antimalarial activity has also been shown in vitro and in vivo.









