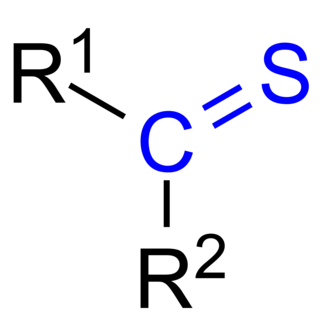The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive elements polonium (Po) and livermorium (Lv). Often, oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from sulfur, selenium, tellurium, and polonium. The word "chalcogen" is derived from a combination of the Greek word khalkόs (χαλκός) principally meaning copper, and the Latinized Greek word genēs, meaning born or produced.

In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis.

Phosphorus is a chemical element; it has symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Earth. It has a concentration in the Earth's crust of about one gram per kilogram. In minerals, phosphorus generally occurs as phosphate.
A period 3 element is one of the chemical elements in the third row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases: a new row is begun when chemical behavior begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block. All of the period 3 elements occur in nature and have at least one stable isotope.

Parathion, also called parathion-ethyl or diethyl parathion and locally known as "Folidol", is an organophosphate insecticide and acaricide. It was originally developed by IG Farben in the 1940s. It is highly toxic to non-target organisms, including humans, so its use has been banned or restricted in most countries. The basic structure is shared by parathion methyl.

Chlorpyrifos (CPS), also known as Chlorpyrifos ethyl, is an organophosphate pesticide that has been used on crops, animals, and buildings, and in other settings, to kill several pests, including insects and worms. It acts on the nervous systems of insects by inhibiting the acetylcholinesterase enzyme. Chlorpyrifos was patented in 1966 by Dow Chemical Company.
In chemistry, a hypervalent molecule is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. Phosphorus pentachloride, sulfur hexafluoride, chlorine trifluoride, the chlorite ion, and the triiodide ion are examples of hypervalent molecules.
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

Phosphorus pentoxide is a chemical compound with molecular formula P4O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent.

In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent.
In organic chemistry, thioketones are organosulfur compounds related to conventional ketones in which the oxygen has been replaced by a sulfur. Instead of a structure of R2C=O, thioketones have the structure R2C=S, which is reflected by the prefix "thio-" in the name of the functional group. Thus the simplest thioketone is thioacetone, the sulfur analog of acetone. Unhindered alkylthioketones typically tend to form polymers or rings.

1,3,2,4-Dithiadiphosphetane 2,4-disulfides are a class of organophosphorus, four-membered ring compounds which contain a P2S2 ring. Many of these compounds are able to act as sources of the dithiophosphine ylides; the most well known example is Lawesson's reagent.
Carbon is a primary component of all known life on Earth, and represents approximately 45–50% of all dry biomass. Carbon compounds occur naturally in great abundance on Earth. Complex biological molecules consist of carbon atoms bonded with other elements, especially oxygen and hydrogen and frequently also nitrogen, phosphorus, and sulfur.

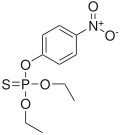
Organothiophosphates or organophosphorothioates are a subclass of organophosphorus compounds and of thiophosphate compounds. They are the organic compounds that contain a phosphate group in which one or more oxygen atoms is substituted by sulfur. Many are used as pesticides, some have medical applications, and some are used as oil additives. They generally have the chemical formula (RO)3PS, [(RO)2P(S)O]−, R(RO)2PS, etc.
Malaoxon (Liromat, Malation oxon, Malthon oxon) is a chemical compound with the formula C10H19O7PS. More specifically, it is a phosphorothioate. It is a breakdown product of, and more toxic than, malathion.
Thiophosphates (or phosphorothioates, PS) are chemical compounds and anions with the general chemical formula PS
4−xO3−
x (x = 0, 1, 2, or 3) and related derivatives where organic groups are attached to one or more O or S. Thiophosphates feature tetrahedral phosphorus(V) centers.
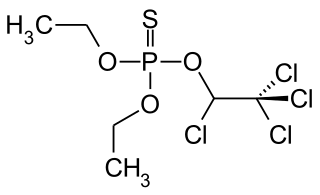
Chlorethoxyfos is an organophosphate acetylcholinesterase inhibitor used as an insecticide. It is registered for the control of corn rootworms, wireworms, cutworms, seed corn maggot, white grubs and symphylans on corn. The insecticide is sold under the trade name Fortress by E.I. du Pont de Nemours & Company.
In chemistry, molecular oxohalides (oxyhalides) are a group of chemical compounds in which both oxygen and halogen atoms are attached to another chemical element A in a single molecule. They have the general formula AOmXn, where X is a halogen. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine (I) in their molecules. The element A may be a main group element, a transition element, a rare earth element or an actinide. The term oxohalide, or oxyhalide, may also refer to minerals and other crystalline substances with the same overall chemical formula, but having an ionic structure.

Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.

EPN is an insecticide of the phosphonothioate class. It is used against pests such as European corn borer, rice stem borer, bollworm, tobacco budworm, and boll weevil.