Related Research Articles

Ribonuclease is a type of nuclease that catalyzes the degradation of RNA into smaller components. Ribonucleases can be divided into endoribonucleases and exoribonucleases, and comprise several sub-classes within the EC 2.7 and 3.1 classes of enzymes.

Dicer, also known as endoribonuclease Dicer or helicase with RNase motif, is an enzyme that in humans is encoded by the DICER1 gene. Being part of the RNase III family, Dicer cleaves double-stranded RNA (dsRNA) and pre-microRNA (pre-miRNA) into short double-stranded RNA fragments called small interfering RNA and microRNA, respectively. These fragments are approximately 20–25 base pairs long with a two-base overhang on the 3′-end. Dicer facilitates the activation of the RNA-induced silencing complex (RISC), which is essential for RNA interference. RISC has a catalytic component Argonaute, which is an endonuclease capable of degrading messenger RNA (mRNA).
DNA glycosylases are a family of enzymes involved in base excision repair, classified under EC number EC 3.2.2. Base excision repair is the mechanism by which damaged bases in DNA are removed and replaced. DNA glycosylases catalyze the first step of this process. They remove the damaged nitrogenous base while leaving the sugar-phosphate backbone intact, creating an apurinic/apyrimidinic site, commonly referred to as an AP site. This is accomplished by flipping the damaged base out of the double helix followed by cleavage of the N-glycosidic bond.
An esterase is a hydrolase enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis.
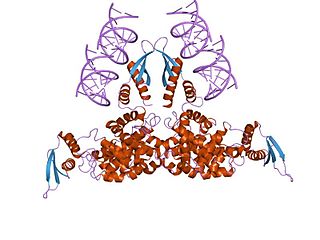
Ribonuclease III (RNase III or RNase C)(BRENDA 3.1.26.3) is a type of ribonuclease that recognizes dsRNA and cleaves it at specific targeted locations to transform them into mature RNAs. These enzymes are a group of endoribonucleases that are characterized by their ribonuclease domain, which is labelled the RNase III domain. They are ubiquitous compounds in the cell and play a major role in pathways such as RNA precursor synthesis, RNA Silencing, and the pnp autoregulatory mechanism.

Drosha is a Class 2 ribonuclease III enzyme that in humans is encoded by the DROSHA gene. It is the primary nuclease that executes the initiation step of miRNA processing in the nucleus. It works closely with DGCR8 and in correlation with Dicer. It has been found significant in clinical knowledge for cancer prognosis and HIV-1 replication.

Ribonuclease T1 (EC 3.1.27.3, guanyloribonuclease, Aspergillus oryzae ribonuclease, RNase N1, RNase N2, ribonuclease N3, ribonuclease U1, ribonuclease F1, ribonuclease Ch, ribonuclease PP1, ribonuclease SA, RNase F1, ribonuclease C2, binase, RNase Sa, guanyl-specific RNase, RNase G, RNase T1, ribonuclease guaninenucleotido-2'-transferase (cyclizing), ribonuclease N3, ribonuclease N1) is a fungal endonuclease that cleaves single-stranded RNA after guanine residues, i.e., on their 3' end; the most commonly studied form of this enzyme is the version found in the mold Aspergillus oryzae. Owing to its specificity for guanine, RNase T1 is often used to digest denatured RNA prior to sequencing. Similar to other ribonucleases such as barnase and RNase A, ribonuclease T1 has been popular for folding studies.

An exoribonuclease is an exonuclease ribonuclease, which are enzymes that degrade RNA by removing terminal nucleotides from either the 5' end or the 3' end of the RNA molecule. Enzymes that remove nucleotides from the 5' end are called 5'-3' exoribonucleases, and enzymes that remove nucleotides from the 3' end are called 3'-5' exoribonucleases.

Pancreatic ribonuclease family is a superfamily of pyrimidine-specific endonucleases found in high quantity in the pancreas of certain mammals and of some reptiles.
An endoribonuclease is a ribonuclease endonuclease. It cleaves either single-stranded or double-stranded RNA, depending on the enzyme. Example includes both single proteins such as RNase III, RNase A, RNase T1, RNase T2 and RNase H and also complexes of proteins with RNA such as RNase P and the RNA-induced silencing complex. Further examples include endoribonuclease XendoU found in frogs (Xenopus).

Intrinsic, or rho-independent termination, is a process in prokaryotes to signal the end of transcription and release the newly constructed RNA molecule. In prokaryotes such as E. coli, transcription is terminated either by a rho-dependent process or rho-independent process. In the Rho-dependent process, the rho-protein locates and binds the signal sequence in the mRNA and signals for cleavage. Contrarily, intrinsic termination does not require a special protein to signal for termination and is controlled by the specific sequences of RNA. When the termination process begins, the transcribed mRNA forms a stable secondary structure hairpin loop, also known as a Stem-loop. This RNA hairpin is followed by multiple uracil nucleotides. The bonds between uracil and adenine are very weak. A protein bound to RNA polymerase (nusA) binds to the stem-loop structure tightly enough to cause the polymerase to temporarily stall. This pausing of the polymerase coincides with transcription of the poly-uracil sequence. The weak adenine-uracil bonds lower the energy of destabilization for the RNA-DNA duplex, allowing it to unwind and dissociate from the RNA polymerase. Overall, the modified RNA structure is what terminates transcription.
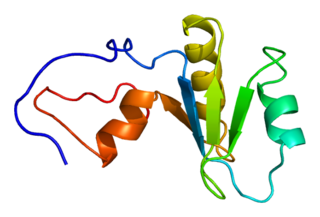
Poly(A)-specific ribonuclease (PARN), also known as polyadenylate-specific ribonuclease or deadenylating nuclease (DAN), is an enzyme that in humans is encoded by the PARN gene.
Ribonuclease T2 is an enzyme. It is a type of endoribonuclease. This enzyme catalyses the following chemical reaction
Ribonuclease IV is an enzyme. This enzyme catalyses the following chemical reaction
Ribonuclease IX is an enzyme. This enzyme catalyses the following chemical reaction
Ribonuclease E is a bacterial ribonuclease that participates in the processing of ribosomal RNA and the chemical degradation of bulk cellular RNA.
Ribonuclease U2 is an enzyme. This enzyme catalyses the following chemical reaction
Ribonuclease F is an enzyme. This enzyme catalyses the following chemical reaction
Ribonuclease V is an enzyme. This enzyme catalyses the following chemical reaction
Double-stranded uracil-DNA glycosylase is an enzyme with systematic name uracil-double-stranded DNA deoxyribohydrolase (uracil-releasing). This enzyme catalyses a specific chemical reaction: it hydrolyses mismatched double-stranded DNA and polynucleotides, releasing free uracil.
References
- ↑ Bachmann M, Trautmann F, Messer R, Zahn RK, Meyer zum Büschenfelde KH, Müller WE (November 1983). "Association of a polyuridylate-specific endoribonuclease with small nuclear ribonucleo-proteins which had been isolated by affinity chromatography using antibodies from a patient with systemic lupus erythematosus". European Journal of Biochemistry. 136 (3): 447–51. doi: 10.1111/j.1432-1033.1983.tb07762.x . PMID 6227485.