
The vomeronasal organ (VNO), or Jacobson's organ, is the paired auxiliary olfactory (smell) sense organ located in the soft tissue of the nasal septum, in the nasal cavity just above the roof of the mouth. The name is derived from the fact that it lies adjacent to the unpaired vomer bone in the nasal septum. It is present and functional in all snakes and lizards, and in many mammals, including cats, dogs, cattle, pigs, and some primates; in humans and horses it is present, but is vestigial and non-functional.
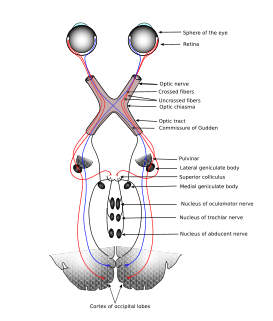
The sensory nervous system is a part of the nervous system responsible for processing sensory information. A sensory system consists of sensory neurons, neural pathways, and parts of the brain involved in sensory perception. Commonly recognized sensory systems are those for vision, hearing, touch, taste, smell, and balance. In short, senses are transducers from the physical world to the realm of the mind where we interpret the information, creating our perception of the world around us.

The olfactory bulb is a neural structure of the vertebrate forebrain involved in olfaction, the sense of smell. It sends olfactory information to be further processed in the amygdala, the orbitofrontal cortex (OFC) and the hippocampus where it plays a role in emotion, memory and learning. The bulb is divided into two distinct structures: the main olfactory bulb and the accessory olfactory bulb. The main olfactory bulb connects to the amygdala via the piriform cortex of the primary olfactory cortex and directly projects from the main olfactory bulb to specific amygdala areas. The accessory olfactory bulb resides on the dorsal-posterior region of the main olfactory bulb and forms a parallel pathway. Destruction of the olfactory bulb results in ipsilateral anosmia, while irritative lesions of the uncus can result in olfactory and gustatory hallucinations.
Stimulus modality, also called sensory modality, is one aspect of a stimulus or what is perceived after a stimulus. For example, the temperature modality is registered after heat or cold stimulate a receptor. Some sensory modalities include: light, sound, temperature, taste, pressure, and smell. The type and location of the sensory receptor activated by the stimulus plays the primary role in coding the sensation. All sensory modalities work together to heighten stimuli sensation when necessary.

The olfactory system, or sense of smell, is the sensory system used for smelling (olfaction). Olfaction is one of the special senses, that have directly associated specific organs. Most mammals and reptiles have a main olfactory system and an accessory olfactory system. The main olfactory system detects airborne substances, while the accessory system senses fluid-phase stimuli.

An olfactory receptor neuron (ORN), also called an olfactory sensory neuron (OSN), is a sensory neuron within the olfactory system.

The olfactory epithelium is a specialized epithelial tissue inside the nasal cavity that is involved in smell. In humans, it measures 9 cm2 and lies on the roof of the nasal cavity about 7 cm above and behind the nostrils. The olfactory epithelium is the part of the olfactory system directly responsible for detecting odors.

The glomerulus is a spherical structure located in the olfactory bulb of the brain where synapses form between the terminals of the olfactory nerve and the dendrites of mitral, periglomerular and tufted cells. Each glomerulus is surrounded by a heterogeneous population of juxtaglomerular neurons and glial cells.
Olfactory receptors (ORs), also known as odorant receptors, are expressed in the cell membranes of olfactory receptor neurons and are responsible for the detection of odorants which give rise to the sense of smell. Activated olfactory receptors trigger nerve impulses which transmit information about odor to the brain. These receptors are members of the class A rhodopsin-like family of G protein-coupled receptors (GPCRs). The olfactory receptors form a multigene family consisting of around 800 genes in humans and 1400 genes in mice.
The docking theory of olfaction proposes that the smell of an odorant molecule is due to a range of weak non-covalent interactions between the odorant [a ligand] and its protein odorant receptor, such as electrostatic and Van der Waals interactions as well as H-bonding, dipole attraction, pi-stacking, metal ion, Cation–pi interaction, and hydrophobic effects, in addition to odorant conformation. While this type of recognition has previously been termed the shape theory of olfaction, which primarily considers molecular shape and size, this latter model is oversimplified since two scent molecules may have similar shapes and sizes but different sets of weak intermolecular forces and therefore activate different combinations of odorant receptors. Earlier “lock and key” and "hand in glove" models of protein−ligand binding has been replaced by a more nuanced pictures which consider the distortion of flexible molecules so as to form the optimal interactions with binding partners as in molecular docking of non-olfactory G-protein coupled receptors.
In medicine and anatomy, the special senses are the senses that have specialized organs devoted to them:

The olfactory tubercle (OT), also known as the tuberculum olfactorium, is a multi-sensory processing center that is contained within the olfactory cortex and ventral striatum and plays a role in reward cognition. The OT has also been shown to play a role in locomotor and attentional behaviors, particularly in relation to social and sensory responsiveness, and it may be necessary for behavioral flexibility. The OT is interconnected with numerous brain regions, especially the sensory, arousal, and reward centers, thus making it a potentially critical interface between processing of sensory information and the subsequent behavioral responses.
A topographic map is the ordered projection of a sensory surface, like the retina or the skin, or an effector system, like the musculature, to one or more structures of the central nervous system. Topographic maps can be found in all sensory systems and in many motor systems.
Hyperosmia is an increased olfactory acuity, usually caused by a lower threshold for odor. This perceptual disorder arises when there is an abnormally increased signal at any point between the olfactory receptors and the olfactory cortex. The causes of hyperosmia may be genetic, hormonal, environmental or the result of benzodiazepine withdrawal syndrome.
Dysosmia is a disorder described as any qualitative alteration or distortion of the perception of smell. Qualitative alterations differ from quantitative alterations, which include anosmia and hyposmia. Dysosmia can be classified as either parosmia or phantosmia. Parosmia is a distortion in the perception of an odorant. Odorants smell different from what one remembers. Phantosmia is the perception of an odor when no odorant is present. The cause of dysosmia still remains a theory. It is typically considered a neurological disorder and clinical associations with the disorder have been made. Most cases are described as idiopathic and the main antecedents related to parosmia are URTIs, head trauma, and nasal and paranasal sinus disease. Dysosmia tends to go away on its own but there are options for treatment for patients that want immediate relief.
Olfactory memory refers to the recollection of odors. Studies have found various characteristics of common memories of odor memory including persistence and high resistance to interference. Explicit memory is typically the form focused on in the studies of olfactory memory, though implicit forms of memory certainly supply distinct contributions to the understanding of odors and memories of them. Research has demonstrated that the changes to the olfactory bulb and main olfactory system following birth are extremely important and influential for maternal behavior. Mammalian olfactory cues play an important role in the coordination of the mother infant bond, and the following normal development of the offspring. Maternal breast odors are individually distinctive, and provide a basis for recognition of the mother by her offspring.
Sniffing is a perceptually-relevant behavior, defined as the active sampling of odors through the nasal cavity for the purpose of information acquisition. This behavior, displayed by all terrestrial vertebrates, is typically identified based upon changes in respiratory frequency and/or amplitude, and is often studied in the context of odor guided behaviors and olfactory perceptual tasks. Sniffing is quantified by measuring intra-nasal pressure or flow or air or, while less accurate, through a strain gauge on the chest to measure total respiratory volume. Strategies for sniffing behavior vary depending upon the animal, with small animals displaying sniffing frequencies ranging from 4 to 12 Hz but larger animals (humans) sniffing at much lower frequencies, usually less than 2 Hz. Subserving sniffing behaviors, evidence for an "olfactomotor" circuit in the brain exists, wherein perception or expectation of an odor can trigger brain respiratory center to allow for the modulation of sniffing frequency and amplitude and thus acquisition of odor information. Sniffing is analogous to other stimulus sampling behaviors, including visual saccades, active touch, and whisker movements in small animals. Atypical sniffing has been reported in cases of neurological disorders, especially those disorders characterized by impaired motor function and olfactory perception.

Insect olfaction refers to the function of chemical receptors that enable insects to detect and identify volatile compounds for foraging, predator avoidance, finding mating partners and locating oviposition habitats. Thus, it is the most important sensation for insects. Most important insect behaviors must be timed perfectly which is dependent on what they smell and when they smell it. For example, olfaction is essential for hunting in many species of wasps, including Polybia sericea.
Retronasal smell, retronasal olfaction, is the ability to perceive flavor dimensions of foods and drinks. Retronasal smell is a sensory modality that produces flavor. It is best described as a combination of traditional smell and taste modalities. Retronasal smell creates flavor from smell molecules in foods or drinks shunting up through the nasal passages as one is chewing. When people use the term "smell", they are usually referring to "orthonasal smell", or the perception of smell molecules that enter directly through the nose and up the nasal passages. Retronasal smell is critical for experiencing the flavor of foods and drinks. Flavor should be contrasted with taste, which refers to five specific dimensions: (1) sweet, (2) salty, (3) bitter, (4) sour, and (5) umami. Perceiving anything beyond these five dimensions, such as distinguishing the flavor of an apple from a pear for example, requires the sense of retronasal smell.
Neurogastronomy is the study of flavor perception and the ways it affects cognition and memory. This interdisciplinary field is influenced by the psychology and neuroscience of sensation, learning, satiety, and decision making. Areas of interest include how olfaction contributes to flavor, food addiction and obesity, taste preferences, and the linguistics of communicating and identifying flavor. The term neurogastronomy was coined by neuroscientist Gordon M. Shepherd.












