Related Research Articles

Endocytosis is a cellular process in which substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a vesicle containing the ingested materials. Endocytosis includes pinocytosis and phagocytosis. It is a form of active transport.

Exocytosis is a form of active transport and bulk transport in which a cell transports molecules out of the cell. As an active transport mechanism, exocytosis requires the use of energy to transport material. Exocytosis and its counterpart, endocytosis, are used by all cells because most chemical substances important to them are large polar molecules that cannot pass through the hydrophobic portion of the cell membrane by passive means. Exocytosis is the process by which a large amount of molecules are released; thus it is a form of bulk transport. Exocytosis occurs via secretory portals at the cell plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structures at the cell plasma membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell.

Clathrin is a protein that plays a role in the formation of coated vesicles. Clathrin was first isolated by Barbara Pearse in 1976. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice that surrounds the vesicle. The protein's name refers to this lattice structure, deriving from Latin clathri meaning lattice. Barbara Pearse named the protein clathrin at the suggestion of Graeme Mitchison, selecting it from three possible options. Coat-proteins, like clathrin, are used to build small vesicles in order to transport molecules within cells. The endocytosis and exocytosis of vesicles allows cells to communicate, to transfer nutrients, to import signaling receptors, to mediate an immune response after sampling the extracellular world, and to clean up the cell debris left by tissue inflammation. The endocytic pathway can be hijacked by viruses and other pathogens in order to gain entry to the cell during infection.
Axon guidance is a subfield of neural development concerning the process by which neurons send out axons to reach their correct targets. Axons often follow very precise paths in the nervous system, and how they manage to find their way so accurately is an area of ongoing research.
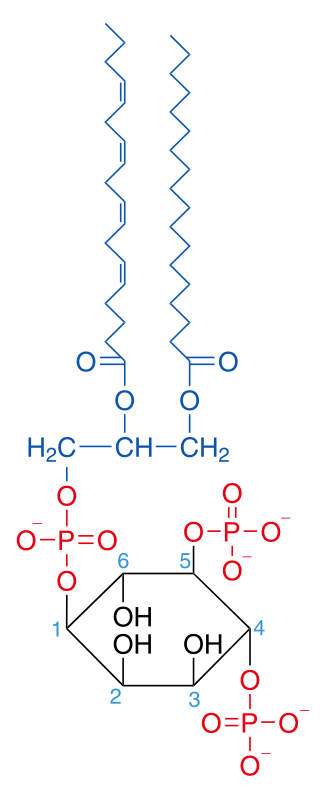
Phosphatidylinositol 4,5-bisphosphate or PtdIns(4,5)P2, also known simply as PIP2 or PI(4,5)P2, is a minor phospholipid component of cell membranes. PtdIns(4,5)P2 is enriched at the plasma membrane where it is a substrate for a number of important signaling proteins. PIP2 also forms lipid clusters that sort proteins.

Phosphatidylinositol (3,4)-bisphosphate is a minor phospholipid component of cell membranes, yet an important second messenger. The generation of PtdIns(3,4)P2 at the plasma membrane activates a number of important cell signaling pathways.
Dynamin is a GTPase responsible for endocytosis in the eukaryotic cell. Dynamin is part of the "dynamin superfamily", which includes classical dynamins, dynamin-like proteins, Mx proteins, OPA1, mitofusins, and GBPs. Members of the dynamin family are principally involved in the scission of newly formed vesicles from the membrane of one cellular compartment and their targeting to, and fusion with, another compartment, both at the cell surface as well as at the Golgi apparatus. Dynamin family members also play a role in many processes including division of organelles, cytokinesis and microbial pathogen resistance.

Eph receptors are a group of receptors that are activated in response to binding with Eph receptor-interacting proteins (Ephrins). Ephs form the largest known subfamily of receptor tyrosine kinases (RTKs). Both Eph receptors and their corresponding ephrin ligands are membrane-bound proteins that require direct cell-cell interactions for Eph receptor activation. Eph/ephrin signaling has been implicated in the regulation of a host of processes critical to embryonic development including axon guidance, formation of tissue boundaries, cell migration, and segmentation. Additionally, Eph/ephrin signaling has been identified to play a critical role in the maintenance of several processes during adulthood including long-term potentiation, angiogenesis, and stem cell differentiation and cancer.

In the nervous system, a synapse is a structure that allows a neuron to pass an electrical or chemical signal to another neuron or a target effector cell. Synapses can be classified as either chemical or electrical, depending on the mechanism of signal transmission between neurons. In the case of electrical synapses, neurons are coupled bidirectionally with each other through gap junctions and have a connected cytoplasmic milieu. These types of synapses are known to produce synchronous network activity in the brain, but can also result in complicated, chaotic network level dynamics. Therefore, signal directionality cannot always be defined across electrical synapses.

Amphiphysin is a protein that in humans is encoded by the AMPH gene.

Cortactin is a monomeric protein located in the cytoplasm of cells that can be activated by external stimuli to promote polymerization and rearrangement of the actin cytoskeleton, especially the actin cortex around the cellular periphery. It is present in all cell types. When activated, it will recruit Arp2/3 complex proteins to existing actin microfilaments, facilitating and stabilizing nucleation sites for actin branching. Cortactin is important in promoting lamellipodia formation, invadopodia formation, cell migration, and endocytosis.

Ephrins are a family of proteins that serve as the ligands of the Eph receptor. Eph receptors in turn compose the largest known subfamily of receptor protein-tyrosine kinases (RTKs).

Dynamin-1 is a protein that in humans is encoded by the DNM1 gene.

Endophilin-A1 is a protein that in humans is encoded by the SH3GL2 gene.

Sorting nexin-9 is a protein that in humans is encoded by the SNX9 gene.

The active zone or synaptic active zone is a term first used by Couteaux and Pecot-Dechavassinein in 1970 to define the site of neurotransmitter release. Two neurons make near contact through structures called synapses allowing them to communicate with each other. As shown in the adjacent diagram, a synapse consists of the presynaptic bouton of one neuron which stores vesicles containing neurotransmitter, and a second, postsynaptic neuron which bears receptors for the neurotransmitter, together with a gap between the two called the synaptic cleft. When an action potential reaches the presynaptic bouton, the contents of the vesicles are released into the synaptic cleft and the released neurotransmitter travels across the cleft to the postsynaptic neuron and activates the receptors on the postsynaptic membrane.
SacI homology domain is most notably found at the amino terminal of the inositol 5'-phosphatase synaptojanin. Synaptic vesicles are recycled with remarkable speed and precision in nerve terminals. A major recycling pathway involves clathrin-mediated endocytosis at endocytic zones located around sites of release. Different 'accessory' proteins linked to this pathway have been shown to alter the shape and composition of lipid membranes, to modify membrane-coat protein interactions, and to influence actin polymerization. These include the GTPase dynamin, the lysophosphatidic acid acyl transferase endophilin, and the phosphoinositide phosphatase synaptojanin.
Neurotransmitters are released into a synapse in packaged vesicles called quanta. One quantum generates a miniature end plate potential (MEPP) which is the smallest amount of stimulation that one neuron can send to another neuron. Quantal release is the mechanism by which most traditional endogenous neurotransmitters are transmitted throughout the body. The aggregate sum of many MEPPs is an end plate potential (EPP). A normal end plate potential usually causes the postsynaptic neuron to reach its threshold of excitation and elicit an action potential. Electrical synapses do not use quantal neurotransmitter release and instead use gap junctions between neurons to send current flows between neurons. The goal of any synapse is to produce either an excitatory postsynaptic potential (EPSP) or an inhibitory postsynaptic potential (IPSP), which generate or repress the expression, respectively, of an action potential in the postsynaptic neuron. It is estimated that an action potential will trigger the release of approximately 20% of an axon terminal's neurotransmitter load.

Synaptic stabilization is crucial in the developing and adult nervous systems and is considered a result of the late phase of long-term potentiation (LTP). The mechanism involves strengthening and maintaining active synapses through increased expression of cytoskeletal and extracellular matrix elements and postsynaptic scaffold proteins, while pruning less active ones. For example, cell adhesion molecules (CAMs) play a large role in synaptic maintenance and stabilization. Gerald Edelman discovered CAMs and studied their function during development, which showed CAMs are required for cell migration and the formation of the entire nervous system. In the adult nervous system, CAMs play an integral role in synaptic plasticity relating to learning and memory.
Clathrin-independent endocytosis refers to the cellular process by which cells internalize extracellular molecules and particles through mechanisms that do not rely on the protein clathrin, playing a crucial role in diverse physiological processes such as nutrient uptake, membrane turnover, and cellular signaling.
References
- 1 2 Montesinos ML, Castellano-Muñoz M, García-Junco-Clemente P, Fernández-Chacón R (September 2005). "Recycling and EH domain proteins at the synapse". Brain Res. Brain Res. Rev. 49 (2): 416–28. doi:10.1016/j.brainresrev.2005.06.002. PMID 16054223. S2CID 20738882.
- ↑ Nemoto Y, Wenk MR, Watanabe M, Daniell L, Murakami T, Ringstad N, Yamada H, Takei K, De Camilli P (November 2001). "Identification and characterization of a synaptojanin 2 splice isoform predominantly expressed in nerve terminals". J. Biol. Chem. 276 (44): 41133–42. doi: 10.1074/jbc.M106404200 . PMID 11498538.
- ↑ Verstreken P, Koh TW, Schulze KL, Zhai RG, Hiesinger PR, Zhou Y, Mehta SQ, Cao Y, Roos J, Bellen HJ (November 2003). "Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating". Neuron. 40 (4): 733–48. doi: 10.1016/S0896-6273(03)00644-5 . PMID 14622578. S2CID 14150492.
- ↑ Torre E, McNiven MA, Urrutia R (December 1994). "Dynamin 1 antisense oligonucleotide treatment prevents neurite formation in cultured hippocampal neurons". J. Biol. Chem. 269 (51): 32411–7. doi: 10.1016/S0021-9258(18)31650-8 . PMID 7798241.
- ↑ Quadri M, Fang M, Picillo M, Olgiati S, Breedveld GJ, Graafland J, Wu B, Xu F, Erro R, Amboni M, Pappatà S, Quarantelli M, Annesi G, Quattrone A, Chien HF, Barbosa ER, Oostra BA, Barone P, Wang J, Bonifati V (2013). "Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset Parkinsonism". Hum. Mutat. 34 (9): 1208–15. doi: 10.1002/humu.22373 . PMID 23804577. S2CID 5715092.
- 1 2 Hopper NA, O'Connor V (May 2005). "Ephrin tempers two-faced synaptojanin 1". Nat. Cell Biol. 7 (5): 454–6. doi:10.1038/ncb0505-454. PMID 15867929. S2CID 19812387.
- ↑ Irie F, Okuno M, Pasquale EB, Yamaguchi Y (May 2005). "EphrinB-EphB signalling regulates clathrin-mediated endocytosis through tyrosine phosphorylation of synaptojanin 1". Nat. Cell Biol. 7 (5): 501–9. doi:10.1038/ncb1252. PMC 1473167 . PMID 15821731.
- 1 2 Tojima T, Akiyama H, Itofusa R, Li Y, Katayama H, Miyawaki A, Kamiguchi H (January 2007). "Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone". Nat. Neurosci. 10 (1): 58–66. doi:10.1038/nn1814. PMID 17159991. S2CID 10762264.
- 1 2 Bonanomi D, Fornasiero EF, Valdez G, Halegoua S, Benfenati F, Menegon A, Valtorta F (November 2008). "Identification of a developmentally regulated pathway of membrane retrieval in neuronal growth cones". J. Cell Sci. 121 (Pt 22): 3757–69. doi:10.1242/jcs.033803. PMC 2731302 . PMID 18940911.
- ↑ Shankland M, Bentley D, Goodman CS (August 1982). "Afferent innervation shapes the dendritic branching pattern of the medial giant interneuron in grasshopper embryos raised in culture". Dev. Biol. 92 (2): 507–20. doi:10.1016/0012-1606(82)90195-6. PMID 7117697.
- ↑ Gong LW, De Camilli P (November 2008). "Regulation of postsynaptic AMPA responses by synaptojanin 1". Proc. Natl. Acad. Sci. U.S.A. 105 (45): 17561–6. Bibcode:2008PNAS..10517561G. doi: 10.1073/pnas.0809221105 . PMC 2579885 . PMID 18987319.