
Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is a volatile liquid. Upon contact with humid air, it forms thick clouds of titanium dioxide and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. It is sometimes referred to as "tickle" or "tickle 4", as a phonetic representation of the symbols of its molecular formula.
An alkyne trimerisation is a [2+2+2] cycloaddition reaction in which three alkyne units react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being applicable to organic synthesis. Being a cycloaddition reaction, it has high atom economy. Many variations have been developed, including cyclisation of mixtures of alkynes and alkenes as well as alkynes and nitriles.
Cycloocta-1,5-diene is a cyclic hydrocarbon with the chemical formula C8H12, specifically [−(CH2)2−CH=CH−]2.

Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.

Tebbe's reagent is the organometallic compound with the formula (C5H5)2TiCH2ClAl(CH3)2. It is used in the methylidenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative. It is a red solid that is pyrophoric in the air, and thus is typically handled with air-free techniques. It was originally synthesized by Fred Tebbe at DuPont Central Research.
Titanium(III) chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known. TiCl3 is one of the most common halides of titanium and is an important catalyst for the manufacture of polyolefins.

Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis, and reactions. Organotitanium compounds in organometallic chemistry contain carbon-titanium chemical bonds. They are reagents in organic chemistry and are involved in major industrial processes.
The Kulinkovich reaction describes the organic synthesis of substituted cyclopropanols through reaction of esters with dialkyldialkoxytitanium reagents, which are generated in situ from Grignard reagents containing a hydrogen in beta-position and titanium(IV) alkoxides such as titanium isopropoxide. This reaction was first reported by Oleg Kulinkovich and coworkers in 1989.

Palladium(II) bis(acetylacetonate) is a compound with formula Pd(C5H7O2)2. This yellow solid is the most common palladium complex of acetylacetonate. This compound is commercially available and used as a catalyst precursor in organic synthesis. The molecule is relatively planar with idealized D2h symmetry.

Nickel(II) bis(acetylacetonate) is a coordination complex with the formula [Ni(acac)2]3, where acac is the anion C5H7O2− derived from deprotonation of acetylacetone. It is a dark green paramagnetic solid that is soluble in organic solvents such as toluene. It reacts with water to give the blue-green diaquo complex Ni(acac)2(H2O)2.
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion (CH
3COCHCOCH−
3) and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCOR′−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. C
5H
7O−
2 in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).

Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, such as palladium chloride.

Titanocene pentasulfide is the organotitanium compound with the formula (C5H5)2TiS5, commonly abbreviated as Cp2TiS5. This metallocene exists as a bright red solid that is soluble in organic solvents. It is of academic interest as a precursor to unusual allotropes of elemental sulfur as well as some related inorganic rings.

Hafnium acetylacetonate, also known as Hf(acac)4, is a coordination compound with formula Hf(C5H7O2)4. This white solid is the main hafnium complex of acetylacetonate. The complex has a square antiprismatic geometry with eight nearly equivalent Hf-O bonds. The molecular symmetry is D2, i.e., the complex is chiral. It is prepared from hafnium tetrachloride and acetylacetone, and base. Zr(acac)4 is very similar in structure and properties.
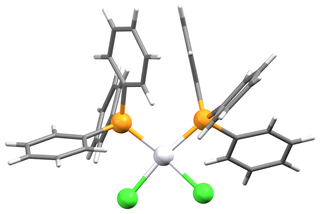
Bis(triphenylphosphine)platinum chloride is a metal phosphine complex with the formula PtCl2[P(C6H5)3]2. Cis- and trans isomers are known. The cis isomer is a white crystalline powder, while the trans isomer is yellow. Both isomers are square planar about the central platinum atom. The cis isomer is used primarily as a reagent for the synthesis of other platinum compounds.
In organic chemistry, the Keck asymmetric allylation is a chemical reaction that involves the nucleophilic addition of an allyl group to an aldehyde. The catalyst is a chiral complex that contains titanium as a Lewis acid. The chirality of the catalyst induces a stereoselective addition, so the secondary alcohol of the product has a predictable absolute stereochemistry based on the choice of catalyst. This name reaction is named for Gary Keck.

Zirconium acetylacetonate is the coordination complex with the formula Zr(C5H7O2)4. It is a common acetylacetonate of zirconium. It is a white solid that exhibits high solubility in nonpolar organic solvents, but not simple hydrocarbons.

Chromium(II) acetylacetonate is the coordination compound with the formula Cr(O2C5H7)2. It is the homoleptic acetylacetonate complex of chromium(II). It is an air-sensitive, paramagnetic yellow brown solid. According to X-ray crystallography, the Cr center is square planar. In contrast to the triplet ground state for this complex, the bis(pyridine) adduct features noninnocent acac2- ligand attached to Cr(III).
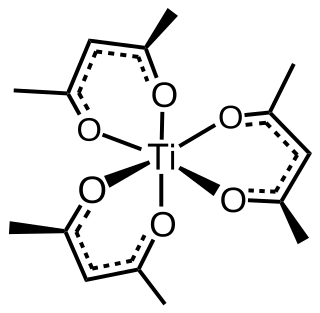
Tris(acetylacetonato)titanium(III), often abbreviated Ti(acac)3, is a coordination complex of titanium(III) featuring acetylacetonate (acac) ligands, making it one of a family of metal acetylacetonates. It is a blue air-sensitive solid that dissolves in nonpolar organic solvents. The compound is prepared by treating titanium trichloride with acetylacetone in the presence of base. Being paramagnetic, it gives a contact-shifted proton NMR signal at 60 ppm upfield of TMS assigned to the methyl group.

Bis(acetylacetonato)iron(II) is a coordination complex of iron with the formula Fe(C5H7O2)2. It can be prepared by reacting iron(II) chloride with 2,4-pentanedione in presence of piperidine.














