Endocytosis is a cellular process in which substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a vesicle containing the ingested material. Endocytosis includes pinocytosis and phagocytosis. It is a form of active transport.

The Golgi apparatus, also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus.
The Coat Protein Complex II, or COPII, is a group of proteins that facilitate the formation of vesicles to transport proteins from the endoplasmic reticulum to the Golgi apparatus or endoplasmic-reticulum–Golgi intermediate compartment. This process is termed anterograde transport, in contrast to the retrograde transport associated with the COPI complex. COPII is assembled in two parts: first an inner layer of Sar1, Sec23, and Sec24 forms; then the inner coat is surrounded by an outer lattice of Sec13 and Sec31.

Endosomes are a collection of intracellular sorting organelles in eukaryotic cells. They are parts of endocytic membrane transport pathway originating from the trans Golgi network. Molecules or ligands internalized from the plasma membrane can follow this pathway all the way to lysosomes for degradation or can be recycled back to the cell membrane in the endocytic cycle. Molecules are also transported to endosomes from the trans Golgi network and either continue to lysosomes or recycle back to the Golgi apparatus.
The Rab family of proteins is a member of the Ras superfamily of small G proteins. Approximately 70 types of Rabs have now been identified in humans. Rab proteins generally possess a GTPase fold, which consists of a six-stranded beta sheet which is flanked by five alpha helices. Rab GTPases regulate many steps of membrane trafficking, including vesicle formation, vesicle movement along actin and tubulin networks, and membrane fusion. These processes make up the route through which cell surface proteins are trafficked from the Golgi to the plasma membrane and are recycled. Surface protein recycling returns proteins to the surface whose function involves carrying another protein or substance inside the cell, such as the transferrin receptor, or serves as a means of regulating the number of a certain type of protein molecules on the surface.
Retromer is a complex of proteins that has been shown to be important in recycling transmembrane receptors from endosomes to the trans-Golgi network (TGN) and directly back to the plasma membrane. Mutations in retromer and its associated proteins have been linked to Alzheimer's and Parkinson's diseases.
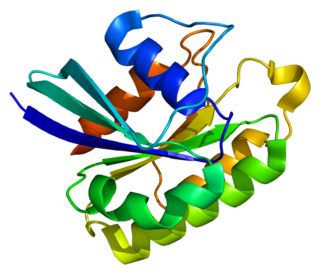
Ras-related protein Rab-5A is a protein that in humans is encoded by the RAB5A gene.
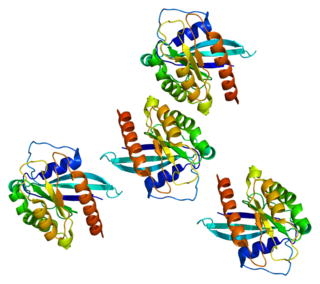
Ras-related protein Rab-7a is a protein that in humans is encoded by the RAB7A gene.

Ras-related protein Rab-11A is a protein that in humans is encoded by the RAB11A gene.
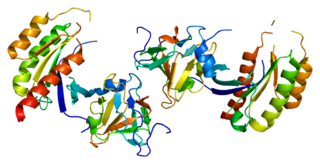
Ras-related protein Rab-8A is a protein that in humans is encoded by the RAB8A gene.

Vesicle-associated membrane protein 3 is a protein that in humans is encoded by the VAMP3 gene.

Rab11 family-interacting protein 1 (Rab11-FIP1) also known as Rab-coupling protein is a protein that in humans is encoded by the RAB11FIP1 gene.

Rab11 family-interacting protein 2 is a protein that in humans is encoded by the RAB11FIP2 gene.

Rab11 family-interacting protein 5 is a protein that in humans is encoded by the RAB11FIP5 gene.

Rab11 family-interacting protein 3 is a protein that in humans is encoded by the RAB11FIP3 gene.

Ras-related protein Rab-22A is a protein that in humans is encoded by the RAB22A gene.

Ras-related protein Rab-25 is a protein that in humans is encoded by the RAB25 gene. It is thought to act as a promoter of tumor development.
The EHD protein family is a relatively small group of proteins which have been shown to play a role in several physiological functions, the most notable being the regulation of endocytotic vesicles. This family is recognized by its highly conserved EH domain, a structural motif that has been shown to facilitate specificity and interaction between protein and ligand. The four mammalian EHD proteins that have been classified are: EHD1, EHD2, EHD3, and EHD4.
Membrane vesicle trafficking in eukaryotic animal cells involves movement of biochemical signal molecules from synthesis-and-packaging locations in the Golgi body to specific release locations on the inside of the plasma membrane of the secretory cell. It takes place in the form of Golgi membrane-bound micro-sized vesicles, termed membrane vesicles (MVs).

Ras-related protein Rab-2B is a protein that in humans is encoded by the RAB2B gene.