
Pseudomonas putida is a Gram-negative, rod-shaped, saprophytic soil bacterium. It has a versatile metabolism and is amenable to genetic manipulation, making it a common organism used in research, bioremediation, and synthesis of chemicals and other compounds.

Acarbose (INN) is an anti-diabetic drug used to treat diabetes mellitus type 2 and, in some countries, prediabetes. It is a generic sold in Europe and China as Glucobay, in North America as Precose, and in Canada as Prandase.
Paenarthrobacter ureafaciens KI72, popularly known as nylon-eating bacteria, is a strain of Paenarthrobacter ureafaciens that can digest certain by-products of nylon 6 manufacture. It uses a set of enzymes to digest nylon, popularly known as nylonase.
The class Flavobacteriia is composed of a single class of environmental bacteria. It contains the family Flavobacteriaceae, which is the largest family in the phylum Bacteroidota. This class is widely distributed in soil, fresh, and seawater habitats. The name is often spelt Flavobacteria, but was officially named Flavobacteriia in 2012.
Paucimonas lemoignei, formerly [Pseudomonas lemoignei], is a Gram-negative soil bacterium. It is aerobic, motile, and rod-shaped.
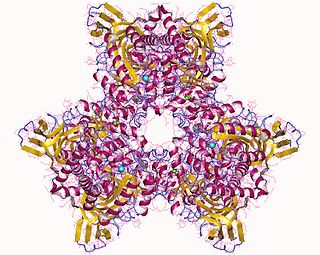
Atrazine Chlorohydrolase (AtzA) is an enzyme (E.C.3.8.1.8), which catalyzes the conversion of atrazine to hydroxyatrazine. Bacterial degradation determines the environmental impact and efficacy of an herbicide or pesticide. Initially, most pesticides are highly effective and show minimal bacterial degradation; however, bacteria can rapidly evolve and gain the ability to metabolize potential nutrients in the environment. Despite a remarkable structural similarity, degradation of atrazine by bacteria capable of melamine degradation was rare; however, since its introduction as a pesticide in the United States, bacteria capable of atrazine degradation have evolved. Currently, Pseudomonas sp. strain ADP seems to be the optimal bacterial strain for atrazine degradations, which appears to be the sole nitrogen source for the bacteria.
Poly(3-hydroxybutyrate) depolymerase (EC 3.1.1.75, PHB depolymerase, systematic name poly[(R)-3-hydroxybutanoate] hydrolase) is an enzyme used in the degradation processes of a natural polyester poly(3-hydroxyburate). This enzyme has growing commercialization interests due to it implications in biodegradable plastic decomposition.
The enzyme tannase (EC 3.1.1.20) catalyzes the following reaction:

Lactonase (EC 3.1.1.81, acyl-homoserine lactonase; systematic name N-acyl-L-homoserine-lactone lactonohydrolase) is a metalloenzyme, produced by certain species of bacteria, which targets and inactivates acylated homoserine lactones (AHLs). It catalyzes the reaction

Paraoxonases are a family of mammalian enzymes with aryldialkylphosphatase activity. There are three paraoxonase isozymes, which were originally discovered for their involvement in the hydrolysis of organophosphates.

Cytophaga is a genus of Gram-negative, gliding, rod-shaped bacteria. This bacterium is commonly found in soil, rapidly digests crystalline cellulose C. hutchinsonii is able to use its gliding motility to move quickly over surfaces. Although the mechanism for this is not known, there is a belief that the flagellum is not used
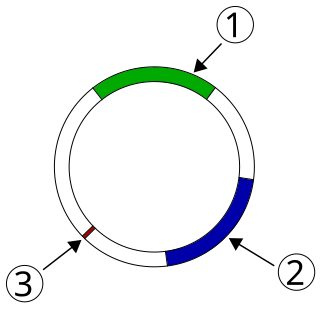
Plasmid-mediated resistance is the transfer of antibiotic resistance genes which are carried on plasmids. Plasmids possess mechanisms that ensure their independent replication as well as those that regulate their replication number and guarantee stable inheritance during cell division. By the conjugation process, they can stimulate lateral transfer between bacteria from various genera and kingdoms. Numerous plasmids contain addiction-inducing systems that are typically based on toxin-antitoxin factors and capable of killing daughter cells that don't inherit the plasmid during cell division. Plasmids often carry multiple antibiotic resistance genes, contributing to the spread of multidrug-resistance (MDR). Antibiotic resistance mediated by MDR plasmids severely limits the treatment options for the infections caused by Gram-negative bacteria, especially family Enterobacteriaceae. The global spread of MDR plasmids has been enhanced by selective pressure from antimicrobial medications used in medical facilities and when raising animals for food.
Organophosphorus acid anhydrolase (OPAA) is an enzyme that been shown to be particularly effective in detoxifying organophosphorus-containing compounds, such as deadly nerve gas used in chemical warfare. The enzyme is found in a diverse range of organisms, including protozoa, squid and clams, mammals, and soil bacteria. A highly active form of the enzyme is typically isolated from the marine bacteria Alteromonas undina for laboratory study. This form is both halophilic and thermophilic, making it particularly useful for detoxification applications. A slightly less active variant of OPAA has also been isolated in mung beans and slime mold duckweed.
The chloride cocaine esterase (EC 3.1.1.84, CocE, hCE2, hCE-2, human carboxylesterase 2; systematic name cocaine benzoylhydrolase) catalyses the reaction
The enzyme pyrethroid hydrolase (EC 3.1.1.88, pyrethroid-hydrolyzing carboxylesterase, pyrethroid-hydrolysing esterase, pyrethroid-hydrolyzing esterase, pyrethroid-selective esterase, pyrethroid-cleaving enzyme, permethrinase, PytH, EstP; systematic name pyrethroid-ester hydrolase) catalyses the reaction
Acidobacterium capsulatum is a bacterium. It is an acidophilic chemoorganotrophic bacterium containing menaquinone. It is gram-negative, facultative anaerobic, mesophilic, non-spore-forming, capsulated, saccharolytic and rod-shaped. It is also motile by peritrichous flagella. Its type strain is JCM 7670.
Pesticide degradation is the process by which a pesticide is transformed into a benign substance that is environmentally compatible with the site to which it was applied. Globally, an estimated 1 to 2.5 million tons of active pesticide ingredients are used each year, mainly in agriculture. Forty percent are herbicides, followed by insecticides and fungicides. Since their initial development in the 1940s, multiple chemical pesticides with different uses and modes of action have been employed. Pesticides are applied over large areas in agriculture and urban settings. Pesticide use, therefore, represents an important source of diffuse chemical environmental inputs.
Sphingobacterium olei is a Gram-stain-negative, rod-shaped, and non-motile bacterium. It was first isolated from oil-contaminated soil in Daqing oil field, China. S. olei has been found to be able to degrade herbicides quizalofop-p-ethyl and diclofop-methyl. Before a name was given, S. olei was designated as strain HAL-9T. The species name olei means "of oil" in Latin.
Cytophaga hutchinsonii is a bacterial species in the genus Cytophaga. C. hutchinsonii is an aerobic, gram-negative, soil, microorganism that exhibits gliding motility, enabling it to move quickly over surfaces and is capable of cellulose degradation.

Plastic degradation in marine bacteria describes when certain pelagic bacteria break down polymers and use them as a primary source of carbon for energy. Polymers such as polyethylene (PE), polypropylene (PP), and polyethylene terephthalate (PET) are incredibly useful for their durability and relatively low cost of production, however it is their persistence and difficulty to be properly disposed of that is leading to pollution of the environment and disruption of natural processes. It is estimated that each year there are 9-14 million metric tons of plastic that are entering the ocean due to inefficient solutions for their disposal. The biochemical pathways that allow for certain microbes to break down these polymers into less harmful byproducts has been a topic of study to develop a suitable anti-pollutant.













