Related Research Articles

In multicellular organisms, stem cells are undifferentiated or partially differentiated cells that can change into various types of cells and proliferate indefinitely to produce more of the same stem cell. They are the earliest type of cell in a cell lineage. They are found in both embryonic and adult organisms, but they have slightly different properties in each. They are usually distinguished from progenitor cells, which cannot divide indefinitely, and precursor or blast cells, which are usually committed to differentiating into one cell type.

A fibroblast is a type of biological cell typically with a spindle shape that synthesizes the extracellular matrix and collagen, produces the structural framework (stroma) for animal tissues, and plays a critical role in wound healing. Fibroblasts are the most common cells of connective tissue in animals.
Transdifferentiation, also known as lineage reprogramming, is the process in which one mature somatic cell is transformed into another mature somatic cell without undergoing an intermediate pluripotent state or progenitor cell type. It is a type of metaplasia, which includes all cell fate switches, including the interconversion of stem cells. Current uses of transdifferentiation include disease modeling and drug discovery and in the future may include gene therapy and regenerative medicine. The term 'transdifferentiation' was originally coined by Selman and Kafatos in 1974 to describe a change in cell properties as cuticle producing cells became salt-secreting cells in silk moths undergoing metamorphosis.
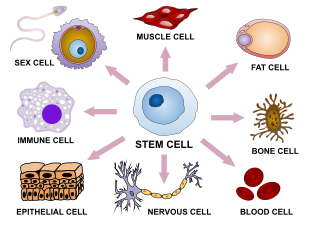
Cellular differentiation is the process in which a stem cell changes from one type to a differentiated one. Usually, the cell changes to a more specialized type. Differentiation happens multiple times during the development of a multicellular organism as it changes from a simple zygote to a complex system of tissues and cell types. Differentiation continues in adulthood as adult stem cells divide and create fully differentiated daughter cells during tissue repair and during normal cell turnover. Some differentiation occurs in response to antigen exposure. Differentiation dramatically changes a cell's size, shape, membrane potential, metabolic activity, and responsiveness to signals. These changes are largely due to highly controlled modifications in gene expression and are the study of epigenetics. With a few exceptions, cellular differentiation almost never involves a change in the DNA sequence itself. However, metabolic composition does get altered quite dramatically where stem cells are characterized by abundant metabolites with highly unsaturated structures whose levels decrease upon differentiation. Thus, different cells can have very different physical characteristics despite having the same genome.
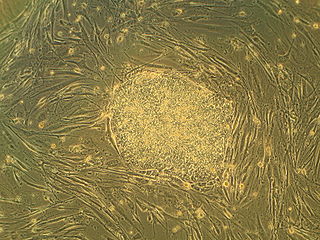
Embryonic stem cells (ESCs) are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre-implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells. Isolating the inner cell mass (embryoblast) using immunosurgery results in destruction of the blastocyst, a process which raises ethical issues, including whether or not embryos at the pre-implantation stage have the same moral considerations as embryos in the post-implantation stage of development.
Organogenesis is the phase of embryonic development that starts at the end of gastrulation and continues until birth. During organogenesis, the three germ layers formed from gastrulation form the internal organs of the organism.

Embryoid bodies (EBs) are three-dimensional aggregates formed by pluripotent stem cells. These include embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC)

Oct-4, also known as POU5F1, is a protein that in humans is encoded by the POU5F1 gene. Oct-4 is a homeodomain transcription factor of the POU family. It is critically involved in the self-renewal of undifferentiated embryonic stem cells. As such, it is frequently used as a marker for undifferentiated cells. Oct-4 expression must be closely regulated; too much or too little will cause differentiation of the cells.

Adult stem cells are undifferentiated cells, found throughout the body after development, that multiply by cell division to replenish dying cells and regenerate damaged tissues. Also known as somatic stem cells, they can be found in juvenile, adult animals, and humans, unlike embryonic stem cells.

A stem cell line is a group of stem cells that is cultured in vitro and can be propagated indefinitely. Stem cell lines are derived from either animal or human tissues and come from one of three sources: embryonic stem cells, adult stem cells, or induced pluripotent stem cells. They are commonly used in research and regenerative medicine.

An organoid is a miniaturised and simplified version of an organ produced in vitro in three dimensions that mimics the key functional, structural, and biological complexity of that organ. It is derived from one or a few cells from a tissue, embryonic stem cells, or induced pluripotent stem cells, which can self-organize in three-dimensional culture owing to their self-renewal and differentiation capacities. The technique for growing organoids has rapidly improved since the early 2010s, and The Scientist named it one of the biggest scientific advancements of 2013. Scientists and engineers use organoids to study development and disease in the laboratory, for drug discovery and development in industry, personalized diagnostics and medicine, gene and cell therapies, tissue engineering, and regenerative medicine.
Neural stem cells (NSCs) are self-renewing, multipotent cells that firstly generate the radial glial progenitor cells that generate the neurons and glia of the nervous system of all animals during embryonic development. Some neural progenitor stem cells persist in highly restricted regions in the adult vertebrate brain and continue to produce neurons throughout life. Differences in the size of the central nervous system are among the most important distinctions between the species and thus mutations in the genes that regulate the size of the neural stem cell compartment are among the most important drivers of vertebrate evolution.

Induced pluripotent stem cells are a type of pluripotent stem cell that can be generated directly from a somatic cell. The iPSC technology was pioneered by Shinya Yamanaka and Kazutoshi Takahashi in Kyoto, Japan, who together showed in 2006 that the introduction of four specific genes, collectively known as Yamanaka factors, encoding transcription factors could convert somatic cells into pluripotent stem cells. Shinya Yamanaka was awarded the 2012 Nobel Prize along with Sir John Gurdon "for the discovery that mature cells can be reprogrammed to become pluripotent."

Cell potency is a cell's ability to differentiate into other cell types. The more cell types a cell can differentiate into, the greater its potency. Potency is also described as the gene activation potential within a cell, which like a continuum, begins with totipotency to designate a cell with the most differentiation potential, pluripotency, multipotency, oligopotency, and finally unipotency.
Cellular cardiomyoplasty, or cell-based cardiac repair, is a new potential therapeutic modality in which progenitor cells are used to repair regions of damaged or necrotic myocardium. The ability of transplanted progenitor cells to improve function within the failing heart has been shown in experimental animal models and in some human clinical trials. In November 2011, a large group of collaborators at Minneapolis Heart Institute Foundation at Abbott Northwestern found no significant difference in left ventricular ejection fraction (LVEF) or other markers, between a group of patients treated with cellular cardiomyoplasty and a group of control patients. In this study, all patients were post MI, post percutaneous coronary intervention (PCI) and that infusion of progenitor cells occurred 2–3 weeks after intervention. In a study that is currently underway, however, more positive results were being reported: In the SCIPIO trial, patients treated with autologous cardiac stem cells post MI have been reported to be showing statistically significant increases in LVEF and reduction in infarct size over the control group at four months after implant. Positive results at the one-year mark are even more pronounced. Yet the SCIPIO trial "was recently called into question". Harvard University is "now investigating the integrity of some of the data". The Lancet recently published a non-specific ‘Expression of concern’ about the paper. Subsequently, another preclinical study also raised doubts on the rationale behind using this special kind of cell, as it was found that the special cells only have a minimal ability in generating new cardiomyocytes. Some specialists therefore now raise concerns to continue.
P19 cells is an embryonic carcinoma cell line derived from an embryo-derived teratocarcinoma in mice. The cell line is pluripotent and can differentiate into cell types of all three germ layers. Also, it is the most characterized embryonic carcinoma (EC) cell line that can be induced into cardiac muscle cells and neuronal cells by different specific treatments. Indeed, exposing aggregated P19 cells to dimethyl sulfoxide (DMSO) induces differentiation into cardiac and skeletal muscle. Also, exposing P19 cells to retinoic acid (RA) can differentiate them into neuronal cells.
Human engineered cardiac tissues (hECTs) are derived by experimental manipulation of pluripotent stem cells, such as human embryonic stem cells (hESCs) and, more recently, human induced pluripotent stem cells (hiPSCs) to differentiate into human cardiomyocytes. Interest in these bioengineered cardiac tissues has risen due to their potential use in cardiovascular research and clinical therapies. These tissues provide a unique in vitro model to study cardiac physiology with a species-specific advantage over cultured animal cells in experimental studies. hECTs also have therapeutic potential for in vivo regeneration of heart muscle. hECTs provide a valuable resource to reproduce the normal development of human heart tissue, understand the development of human cardiovascular disease (CVD), and may lead to engineered tissue-based therapies for CVD patients.
Gordon M. Keller is a Canadian scientist recognized for his research on applying developmental biology findings to in vitro pluripotent stem cell differentiation. He is currently a Senior Scientist at the Ontario Cancer Institute, a Professor at the University of Toronto and the director of the McEwen Centre for Regenerative Medicine.

Christine L. Mummery (1953) is an appointed professor of Developmental Biology at Leiden University and the head of the Department of Anatomy and Embryology at Leiden University Medical Center in the Netherlands.
A myelinoid or myelin organoid is a three dimensional in vitro cultured model derived from human pluripotent stem cells (hPSCs) that represents various brain regions, the spinal cord or the peripheral nervous system in early fetal human development. Myelinoids have the capacity to recapitulate aspects of brain developmental processes, microenvironments, cell to cell interaction, structural organization and cellular composition. The differentiating aspect dictating whether an organoid is deemed a cerebral organoid/brain organoid or myelinoid is the presence of myelination and compact myelin formation that is a defining feature of myelinoids. Due to the complex nature of the human brain, there is a need for model systems which can closely mimic complicated biological processes. Myelinoids provide a unique in vitro model through which myelin pathology, neurodegenerative diseases, developmental processes and therapeutic screening can be accomplished.
References
- 1 2 3 4 5 6 7 8 9 10 11 Cohen DE, Melton D (2011). "Turning straw into gold: directing cell fate for regenerative medicine". Nature Reviews Genetics. 12 (4): 243–252. doi:10.1038/nrg2938. PMID 21386864. S2CID 26358726.
- 1 2 3 4 Murry CE, Keller G (2008). "Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development". Cell. 132 (4): 661–680. doi: 10.1016/j.cell.2008.02.008 . PMID 18295582.
- ↑ Wichterle H, Lieberam I, Porter JA, Jessell TM (2002). "Directed differentiation of embryonic stem cells into motor neurons". Cell. 110 (3): 385–397. doi: 10.1016/S0092-8674(02)00835-8 . PMID 12176325.
- 1 2 3 4 5 Spagnoli FM, Hemmati-Brivanlou A (2006). "Guiding embryonic stem cells towards differentiation: lessons from molecular embryology". Current Opinion in Genetics & Development. 16 (5): 469–475. doi:10.1016/j.gde.2006.08.004. PMID 16919445.
- 1 2 3 4 5 6 Keller G (2005). "Embryonic stem cell differentiation: emergence of a new era in biology and medicine". Genes & Development. 19 (10). genesdev.cshlp.org: 1129–1155. doi: 10.1101/gad.1303605 . PMID 15905405 . Retrieved 2014-11-06.
- 1 2 3 4 5 6 Sterneckert JL, Reinhardt P, Schöler HR (2014). "Investigating human disease using stem cell models". Nature Reviews Genetics. 15 (9): 625–639. doi:10.1038/nrg3764. PMID 25069490. S2CID 22976547.
- ↑ Jones-Villeneuve EM, McBurney MW, Rogers KA, Kalnins VI (1982). "Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells". The Journal of Cell Biology. 94 (2). The Rockefeller University Press: 253–262. doi:10.1083/jcb.94.2.253. PMC 2112882 . PMID 7107698.
- 1 2 3 4 Nishikawa S, Jakt LM, Era T (2007). "Embryonic stem-cell culture as a tool for developmental cell biology". Nature Reviews Molecular Cell Biology. 8 (6): 502–507. doi:10.1038/nrm2189. PMID 17522593. S2CID 205494131.
- ↑ Davis RL, Weintraub H, Lassar AB (1987). "Expression of a single transfected cDNA converts fibroblasts to myoblasts". Cell. 51 (6): 987–1000. doi:10.1016/0092-8674(87)90585-X. PMID 3690668. S2CID 37741454.
- ↑ Marchetti S, Gimond C, Iljin K, Bourcier C, Alitalo K, Pouysségur J, Pagès G (2002). "Endothelial cells genetically selected from differentiating mouse embryonic stem cells incorporate at sites of neovascularization in vivo". Journal of Cell Science. 115 (Pt 10): 2075–2085. doi:10.1242/jcs.115.10.2075. PMID 11973349.
- ↑ Klug MG, Soonpaa MH, Koh GY, Field LJ (1996). "Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts". Journal of Clinical Investigation. 98 (1): 216–224. doi:10.1172/JCI118769. PMC 507419 . PMID 8690796.
- ↑ Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R (2001). "Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets". Science. 292 (5520): 1389–1394. Bibcode:2001Sci...292.1389L. doi:10.1126/science.1058866. PMID 11326082. S2CID 13025470.
- ↑ Chal J, Oginuma M, Al Tanoury Z, Gobert B, Sumara O, Hick A, Bousson F, Zidouni Y, Mursch C, Moncuquet P, Tassy O, Vincent S, Miyanari A, Bera A, Garnier JM, Guevara G, Hestin M, Kennedy L, Hayashi S, Drayton B, Cherrier T, Gayraud-Morel B, Gussoni E, Relaix F, Tajbakhsh S, Pourquié O (August 2015). "Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy" (PDF). Nature Biotechnology . 33 (9): 962–9. doi:10.1038/nbt.3297. PMID 26237517. S2CID 21241434.

- ↑ Shelton M, Kocharyan A, Liu J, Skerjanc IS, Stanford WL (2016). "Robust generation and expansion of skeletal muscle progenitors and myocytes from human pluripotent stem cells". Methods. 101: 73–84. doi: 10.1016/j.ymeth.2015.09.019 . PMID 26404920.

- ↑ "First test of human embryonic stem cell therapy in people discontinued - The Washington Post". washingtonpost.com. Retrieved 2014-11-06.
- ↑ "Japanese team first to use iPS cells in bid to restore human sight | The Japan Times". japantimes.co.jp. Retrieved 2014-11-06.[ permanent dead link ]
