Related Research Articles

An electric current is a flow of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is defined as the net rate of flow of electric charge through a surface. The moving particles are called charge carriers, which may be one of several types of particles, depending on the conductor. In electric circuits the charge carriers are often electrons moving through a wire. In semiconductors they can be electrons or holes. In an electrolyte the charge carriers are ions, while in plasma, an ionized gas, they are ions and electrons.

Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

Mechanical engineering is the study of physical machines that may involve force and movement. It is an engineering branch that combines engineering physics and mathematics principles with materials science, to design, analyze, manufacture, and maintain mechanical systems. It is one of the oldest and broadest of the engineering branches.
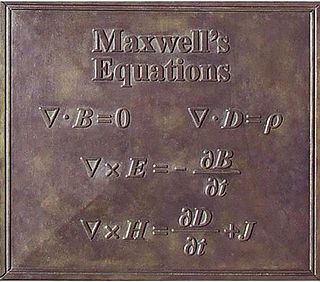
Maxwell's equations, or Maxwell–Heaviside equations, are a set of coupled partial differential equations that, together with the Lorentz force law, form the foundation of classical electromagnetism, classical optics, electric and magnetic circuits. The equations provide a mathematical model for electric, optical, and radio technologies, such as power generation, electric motors, wireless communication, lenses, radar, etc. They describe how electric and magnetic fields are generated by charges, currents, and changes of the fields. The equations are named after the physicist and mathematician James Clerk Maxwell, who, in 1861 and 1862, published an early form of the equations that included the Lorentz force law. Maxwell first used the equations to propose that light is an electromagnetic phenomenon. The modern form of the equations in their most common formulation is credited to Oliver Heaviside.

Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology.

Voltage, also known as (electrical) potential difference, electric pressure, or electric tension is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to move a positive test charge from the first point to the second point. In the International System of Units (SI), the derived unit for voltage is the volt (V).

Ohm's law states that the electric current through a conductor between two points is directly proportional to the voltage across the two points. Introducing the constant of proportionality, the resistance, one arrives at the three mathematical equations used to describe this relationship:
In thermodynamics, dissipation is the result of an irreversible process that affects a thermodynamic system. In a dissipative process, energy transforms from an initial form to a final form, where the capacity of the final form to do thermodynamic work is less than that of the initial form. For example, transfer of energy as heat is dissipative because it is a transfer of energy other than by thermodynamic work or by transfer of matter, and spreads previously concentrated energy. Following the second law of thermodynamics, in conduction and radiation from one body to another, the entropy varies with temperature, but never decreases in an isolated system.
Many letters of the Latin alphabet, both capital and small, are used in mathematics, science, and engineering to denote by convention specific or abstracted constants, variables of a certain type, units, multipliers, or physical entities. Certain letters, when combined with special formatting, take on special meaning.
This is an alphabetical list of articles pertaining specifically to mechanical engineering. For a broad overview of engineering, please see List of engineering topics. For biographies please see List of engineers.
This is an alphabetical list of articles pertaining specifically to electrical and electronics engineering. For a thematic list, please see List of electrical engineering topics. For a broad overview of engineering, see List of engineering topics. For biographies, see List of engineers.

Analogical models are a method of representing a phenomenon of the world, often called the "target system" by another, more understandable or analysable system. They are also called dynamical analogies.
This is an alphabetical list of articles pertaining specifically to Engineering Science and Mechanics (ESM). For a broad overview of engineering, please see Engineering. For biographies please see List of engineers and Mechanicians.

Solid is one of the four fundamental states of matter along with liquid, gas, and plasma. The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice, or irregularly. Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.
This glossary of engineering terms is a list of definitions about the major concepts of engineering. Please see the bottom of the page for glossaries of specific fields of engineering.
This glossary of physics is a list of definitions of terms and concepts relevant to physics, its sub-disciplines, and related fields, including mechanics, materials science, nuclear physics, particle physics, and thermodynamics. For more inclusive glossaries concerning related fields of science and technology, see Glossary of chemistry terms, Glossary of astronomy, Glossary of areas of mathematics, and Glossary of engineering.
Most of the terms listed in Wikipedia glossaries are already defined and explained within Wikipedia itself. However, glossaries like this one are useful for looking up, comparing and reviewing large numbers of terms together. You can help enhance this page by adding new terms or writing definitions for existing ones.
This glossary of electrical and electronics engineering is a list of definitions of terms and concepts related specifically to electrical engineering and electronics engineering. For terms related to engineering in general, see Glossary of engineering.
This glossary of engineering terms is a list of definitions about the major concepts of engineering. Please see the bottom of the page for glossaries of specific fields of engineering.
References
- ↑ Smaller Instruments and Appliances: The Abney Level and Clinometer, A Manual of the Principal Instruments used in American Engineering and Surveying, W. & L. E. Gurley, Troy, NY, 1891; page 219.
- ↑ George William Usill, Clinometers: The Abney Level, Practical Surveying, Crosby Lockwood and Son, London, 1889; page 33.
- 1 2 Punmia, Dr B. C.; Jain, Ashok Kumar; Jain, Arun Kr (2003-05-01). Basic Civil Engineering. Firewall Media. ISBN 9788170084037.
- ↑ Abrams law, air and high water-to-cement ratios by ELSEVIER
- 1 2 "ASABE". www.asabe.org. Retrieved 2018-04-13.
- ↑ Scott, John S. (1992-10-31). Dictionary Of Civil Engineering. Springer Science & Business Media. ISBN 9780412984211.
- ↑ IUPAC Gold Book – absolute electrode potential
- ↑ "Unit of thermodynamic temperature (kelvin)". SI Brochure, 8th edition. Bureau International des Poids et Mesures. 13 March 2010 [1967]. Section 2.1.1.5. Archived from the original on 7 October 2014. Retrieved 20 June 2017.Note: The triple point of water is 0.01 °C, not 0 °C; thus 0 K is −273.15 °C, not −273.16 °C.
- ↑ Arora, C. P. (2001). Thermodynamics. Tata McGraw-Hill. Table 2.4 page 43. ISBN 978-0-07-462014-4.
- ↑ Zielinski, Sarah (1 January 2008). "Absolute Zero". Smithsonian Institution. Archived from the original on 2013-04-01. Retrieved 2012-01-26.
- ↑ IUPAC, Compendium of Chemical Terminology , 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Absorbance". doi:10.1351/goldbook.A00028
- ↑ "Glossary - "Abutment"". U.S. Bureau of Reclamation. Archived from the original on 25 December 2007. Retrieved 24 January 2015.
- ↑ Crew, Henry (2008). The Principles of Mechanics. BiblioBazaar, LLC. p. 43. ISBN 978-0-559-36871-4.
- ↑ Bondi, Hermann (1980). Relativity and Common Sense. Courier Dover Publications. pp. 3. ISBN 978-0-486-24021-3.
- ↑ Lehrman, Robert L. (1998). Physics the Easy Way. Barron's Educational Series. pp. 27. ISBN 978-0-7641-0236-3.
- ↑ IUPAC Gold Book - acid
- ↑ Dictionary of architectural and building technology. London: E & F N Spon. 1998. p. 3. ISBN 0-419-22280-4.
- ↑ Derek Butterfield; Alf Fulcher; Rhodes, Brian; Stewart, Bill; Derick Tickle; Windsor, John C. (2005). Painting and Decorating: An Information Manual. Blackwell/Futura. p. 145. ISBN 1-4051-1254-9.
- ↑ "About Actuators". www.thomasnet.com. Archived from the original on 2016-05-08. Retrieved 2016-04-26.
- ↑ Carathéodory, C. (1909). "Untersuchungen über die Grundlagen der Thermodynamik". Mathematische Annalen. 67 (3): 355–386. doi:10.1007/BF01450409. S2CID 118230148.. A translation may be found here. Also a mostly reliable translation is to be found in Kestin, J. (1976). The Second Law of Thermodynamics. Stroudsburg, PA: Dowden, Hutchinson & Ross.
- ↑ Bailyn, M. (1994). A Survey of Thermodynamics. New York, NY: American Institute of Physics Press. p. 21. ISBN 0-88318-797-3.
- ↑ "Aerobic Diestion" (PDF). Water Environment Federation. Archived from the original (PDF) on 27 March 2016. Retrieved 19 March 2016.
- ↑ "Handbook Biological Wastewater Treatment - Design of Activated Sludge Systems" . Retrieved 19 March 2016.
- ↑ "Aerobic Waste Digesters" . Retrieved 17 March 2017.
- ↑ Daniel Malacara, Zacarias Malacara, Handbook of optical design. Page 379
- ↑ See Herstein, I. N. (1964). Topics in Algebra. Ginn and Company. ISBN 0-471-02371-X., page 1: "An algebraic system can be described as a set of objects together with some operations for combining them".
- ↑ See (Herstein 1964) , page 1: "...it also serves as the unifying thread which interlaces almost all of mathematics".
- ↑ "IUPAC Gold Book - alkanes". IUPAC. March 27, 2017. doi:10.1351/goldbook.A00222 . Retrieved 2018-08-23.
- ↑ Wade, L.G. (2006). Organic Chemistry (6th ed.). Pearson Prentice Hall. p. 279. ISBN 978-1-4058-5345-3.
- ↑ Alkyne. Encyclopædia Britannica
- ↑ Callister, W. D. "Materials Science and Engineering: An Introduction" 2007, 7th edition, John Wiley and Sons, Inc. New York, Section 4.3 and Chapter 9.
- ↑ N. N. Bhargava & D. C. Kulshreshtha (1983). Basic Electronics & Linear Circuits. Tata McGraw-Hill Education. p. 90. ISBN 978-0-07-451965-3.
- ↑ National Electric Light Association (1915). Electrical meterman's handbook. Trow Press. p. 81.
- ↑ "Amino". Dictionary.com. 2015. Retrieved 3 July 2015.
- ↑ "amino acid". Cambridge Dictionaries Online. Cambridge University Press. 2015. Retrieved 3 July 2015.
- ↑ "amino". FreeDictionary.com. Farlex. 2015. Retrieved 3 July 2015.
- ↑ SI supports only the use of symbols and deprecates the use of abbreviations for units. "Bureau International des Poids et Mesures" (PDF). 2006. p. 130. Archived (PDF) from the original on 21 June 2007. Retrieved 21 November 2011.
- ↑ "2.1. Unit of electric current (ampere)", SI brochure (8th ed.), BIPM, archived from the original on 3 February 2012, retrieved 19 November 2011
- ↑ Base unit definitions: Ampere Archived 25 April 2017 at the Wayback Machine Physics.nist.gov. Retrieved on 2010-09-28.
- ↑ IUPAC, Compendium of Chemical Terminology , 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "amphoteric". doi:10.1351/goldbook.A00306
- ↑ Crecraft, David; Gorham, David (2003). Electronics, 2nd Ed. CRC Press. p. 168. ISBN 978-0748770366.
- ↑ Agarwal, Anant; Lang, Jeffrey (2005). Foundations of Analog and Digital Electronic Circuits. Morgan Kaufmann. p. 331. ISBN 978-0080506814.
- ↑ Glisson, Tildon H. (2011). Introduction to Circuit Analysis and Design. Springer Science and Business Media. ISBN 978-9048194438.
- ↑ "Angular Velocity and Acceleration". Theory.uwinnipeg.ca. Archived from the original on 2012-02-22. Retrieved 2015-04-13.
- ↑ University of Colorado Boulder (November 21, 2013). "Atoms and Elements, Isotopes and Ions". colorado.edu.
- ↑ "What is buoyant force? (Article) | Fluids".
- ↑ Acott, Chris (1999). "The diving "Law-ers": A brief resume of their lives". South Pacific Underwater Medicine Society Journal . 29 (1). ISSN 0813-1988. OCLC 16986801. Archived from the original on April 2, 2011. Retrieved 2009-06-13.
{{cite journal}}: CS1 maint: unfit URL (link) - ↑ "architecture". Encyclopedia Britannica. Retrieved 2017-10-27.
- ↑ Groover, Mikell (2014). Fundamentals of Modern Manufacturing: Materials, Processes, and Systems.
- ↑ Rifkin, Jeremy (1995). The End of Work: The Decline of the Global Labor Force and the Dawn of the Post-Market Era. Putnam Publishing Group. pp. 66, 75. ISBN 978-0-87477-779-6.
- ↑ Automaton - Definition and More from the Free Merriam-Webster Dictionary http://www.merriam-webster.com/dictionary/automaton
- ↑ Williams, Jan R.; Susan F. Haka; Mark S. Bettner; Joseph V. Carcello (2008). Financial & Managerial Accounting. McGraw-Hill Irwin. p. 40. ISBN 978-0-07-299650-0.
- ↑ Crompton, T.R. (2000-03-20). Battery Reference Book (third ed.). Newnes. p. Glossary 3. ISBN 978-0-08-049995-6 . Retrieved 2016-03-18.
- ↑ Pauling, Linus (1988). "15: Oxidation-Reduction Reactions; Electrolysis.". General Chemistry . New York: Dover Publications, Inc. p. 539. ISBN 978-0-486-65622-9.
- ↑ Pistoia, Gianfranco (2005-01-25). Batteries for Portable Devices. Elsevier. p. 1. ISBN 978-0-08-045556-3 . Retrieved 2016-03-18.
- ↑ Gere, J.M.; Timoshenko, S.P. (1996), Mechanics of Materials:Forth edition, Nelson Engineering, ISBN 0534934293
- ↑ Beer, F.; Johnston, E.R. (1984), Vector mechanics for engineers: statics, McGraw Hill, pp. 62–76
- ↑ Clancy, L. J. (1975). Aerodynamics. Wiley. ISBN 978-0-470-15837-1.
- ↑ Batchelor, G. K. (2000). An Introduction to Fluid Dynamics. Cambridge: University Press. ISBN 978-0-521-66396-0.
- ↑ Silberberg, Martin S. (2009). Chemistry: the molecular nature of matter and change (5th ed.). Boston: McGraw-Hill. p. 206. ISBN 9780073048598.
- ↑ J. Dalton (1802), "Essay IV. On the expansion of elastic fluids by heat," Memoirs of the Literary and Philosophical Society of Manchester, vol. 5, pt. 2, pages 595–602; see page 600.
- ↑ "Major: Engineering Physics". The Princeton Review. 2017. p. 01. Retrieved June 4, 2017.
- ↑ "Introduction" (online). Princeton University . Retrieved June 26, 2011.
- ↑ Khare, P.; A. Swarup (26 January 2009). Engineering Physics: Fundamentals & Modern Applications (13th ed.). Jones & Bartlett Learning. pp. xiii–Preface. ISBN 978-0-7637-7374-8.
- ↑ Mukherji, Uma (2003). Engineering Physics (online). Alpha Science. ISBN 9781842650646 . Retrieved June 26, 2011– via Internet Archive.
Engineering Physics.
- ↑ Krigger, John; Chris Dorsi (2004). Residential Energy: Cost Savings and Comfort for Existing Buildings. Helena, Montana: Saturn Resource Management. p. 110. ISBN 1-880120-12-7. OCLC 56315804.
- ↑ International Bureau of Weights and Measures (2006), The International System of Units (SI) (PDF) (8th ed.), p. 120, ISBN 92-822-2213-6, archived (PDF) from the original on 2021-06-04, retrieved 2021-12-16
- ↑ American Heritage Dictionary of the English Language, Online Edition (2009). Houghton Mifflin Co., hosted by Yahoo! Education.
- ↑ The American Heritage Dictionary, Second College Edition (1985). Boston: Houghton Mifflin Co., p. 691.
- ↑ McGraw-Hill Dictionary of Physics, Fifth Edition (1997). McGraw-Hill, Inc., p. 224.
- ↑ Plesha, Michael E.; Gray, Gary L.; Costanzo, Francesco (2013). Engineering Mechanics: Statics (2nd ed.). New York: McGraw-Hill Companies Inc. pp. 364–407. ISBN 978-0-07-338029-2.
- ↑ A Guide to Zero Defects: Quality and Reliability Assurance Handbook. Washington, D.C.: Office of the Assistant Secretary of Defense (Manpower Installations and Logistics). 1965. p. 3. OCLC 7188673. 4155.12-H. Archived from the original on May 29, 2014. Retrieved May 29, 2014.
Early in 1964 the Assistant Secretary of Defense (Installations and Logistics) invited the attention of the Military Departments and the Defense Supply Agency to the potential of Zero Defects. This gave the program substantial impetus. Since that time Zero Defects has been adopted by numerous industrial and Department of Defense activities.
- ↑ Carathéodory, C. (1909).
