
Potassium hydroxide is an inorganic compound with the formula KOH, and is commonly called caustic potash.

Praseodymium is a chemical element; it has symbol Pr and the atomic number 59. It is the third member of the lanthanide series and is considered one of the rare-earth metals. It is a soft, silvery, malleable and ductile metal, valued for its magnetic, electrical, chemical, and optical properties. It is too reactive to be found in native form, and pure praseodymium metal slowly develops a green oxide coating when exposed to air.

Praseodymium(III) chloride is the inorganic compound with the formula PrCl3. Like other lanthanide trichlorides, it exists both in the anhydrous and hydrated forms. It is a blue-green solid that rapidly absorbs water on exposure to moist air to form a light green heptahydrate.

Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula AlCl3. It forms a hexahydrate with the formula [Al(H2O)6]Cl3, containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour.

Iron(II) hydroxide or ferrous hydroxide is an inorganic compound with the formula Fe(OH)2. It is produced when iron(II) salts, from a compound such as iron(II) sulfate, are treated with hydroxide ions. Iron(II) hydroxide is a white solid, but even traces of oxygen impart a greenish tinge. The air-oxidised solid is sometimes known as "green rust".
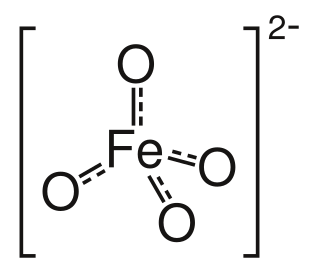
Ferrate(VI) is the inorganic anion with the chemical formula [FeO4]2−. It is photosensitive, contributes a pale violet colour to compounds and solutions containing it and is one of the strongest water-stable oxidizing species known. Although it is classified as a weak base, concentrated solutions containing ferrate(VI) are corrosive and attack the skin and are only stable at high pH. It is similar to the somewhat more stable permanganate.

Beryllium hydroxide, Be(OH)2, is an amphoteric hydroxide, dissolving in both acids and alkalis. Industrially, it is produced as a by-product in the extraction of beryllium metal from the ores beryl and bertrandite. The natural pure beryllium hydroxide is rare (in form of the mineral behoite, orthorhombic) or very rare (clinobehoite, monoclinic). When alkali is added to beryllium salt solutions the α-form (a gel) is formed. If this left to stand or boiled, the rhombic β-form precipitates. This has the same structure as zinc hydroxide, Zn(OH)2, with tetrahedral beryllium centers.
Basic oxides are oxides that show basic properties, in opposition to acidic oxides. A basic oxide can either react with water to form a base, or with an acid to form a salt and water in a neutralization reaction.

Nickel(II) hydroxide is the inorganic compound with the formula Ni(OH)2. It is a lime-green solid that dissolves with decomposition in ammonia and amines and is attacked by acids. It is electroactive, being converted to the Ni(III) oxy-hydroxide, leading to widespread applications in rechargeable batteries.

Cadmium hydroxide is an inorganic compound with the formula Cd(OH)2. It is a white crystalline ionic compound that is a key component of nickel–cadmium battery.
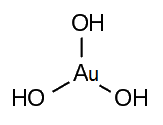
Gold(III) hydroxide, gold trihydroxide, or gold hydroxide is an inorganic compound, a hydroxide of gold, with formula Au(OH)3. It is also called auric acid with formula H3AuO3. It is easily dehydrated above 140 °C to gold(III) oxide. Salts of auric acid are termed aurates.

Praseodymium(IV) oxide is an inorganic compound with chemical formula PrO2.

Cerium(IV) hydroxide, also known as ceric hydroxide, is an inorganic compound with the chemical formula Ce(OH)4. It is a yellowish powder that is insoluble in water but soluble in concentrated acids.

Neodymium(III) hydroxide is an insoluble inorganic compound with the chemical formula Nd(OH)3.

Europium(III) hydroxide is an inorganic compound with a chemical formula Eu(OH)3.
Yttrium(III) hydroxide is an inorganic compound and an alkali with the chemical formula Y(OH)3.
Praseodymium compounds are compounds formed by the lanthanide metal praseodymium (Pr). In these compounds, praseodymium generally exhibits the +3 oxidation state, such as PrCl3, Pr(NO3)3 and Pr(CH3COO)3. However, compounds with praseodymium in the +2 and +4 oxidation states, and unlike other lanthanides, the +5 oxidation state, are also known.

Praseodymium(III) iodide is an inorganic salt, consisting of the rare-earth metal praseodymium and iodine, with the chemical formula PrI3. It forms green crystals. It is soluble in water.
Praseodymium(III) carbonate is an inorganic compound, with a chemical formula of Pr2(CO3)3. The anhydrous form is olive green, and many of its hydrates such as heptahydrate and octahydrate are known. They are all insoluble in water.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.
















