
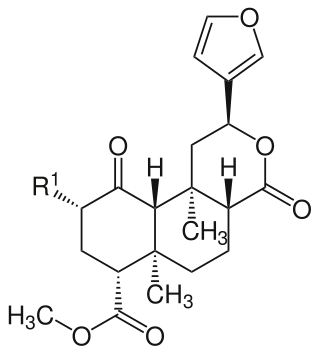
Erinacines are natural substances isolated from Hericium erinaceus . They belong to the group of cyathin diterpenoids (erinacines A-K, P, Q, S, U) and are subjects of pharmacological research.

Erinacines are natural substances isolated from Hericium erinaceus . They belong to the group of cyathin diterpenoids (erinacines A-K, P, Q, S, U) and are subjects of pharmacological research.
Erinacine A, isolated from the cultured mycelia of Hericium erinaceus , the main representative of this compounds group, has an enhancing effect on nerve growth factor synthesis in vitro . [1] It also increases catecholamine in the central nervous system of rats.[ citation needed ]
Erinacine A has also been prepared by total synthesis. [2]
Erinacine E is a kappa opioid receptor agonist. [3]

Salvinorin A is the main active psychotropic molecule in Salvia divinorum. Salvinorin A is considered a dissociative hallucinogen.
Opioid receptors are a group of inhibitory G protein-coupled receptors with opioids as ligands. The endogenous opioids are dynorphins, enkephalins, endorphins, endomorphins and nociceptin. The opioid receptors are ~40% identical to somatostatin receptors (SSTRs). Opioid receptors are distributed widely in the brain, in the spinal cord, on peripheral neurons, and digestive tract.
Salvinorins are a group of natural chemical compounds and their structural analogs. Several salvinorins have been isolated from Salvia divinorum. They are classified as diterpenoid furanolactones. Salvinorin A is a hallucinogen with dissociative effects.

The κ-opioid receptor or kappa opioid receptor, abbreviated KOR or KOP for its ligand ketazocine, is a G protein-coupled receptor that in humans is encoded by the OPRK1 gene. The KOR is coupled to the G protein Gi/G0 and is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering nociception, consciousness, motor control, and mood. Dysregulation of this receptor system has been implicated in alcohol and drug addiction.

Hericium erinaceus, commonly known as lion's mane, yamabushitake, bearded tooth fungus, or bearded hedgehog, is a species of tooth fungus. It tends to grow in a single clump with dangling spines longer than 1 centimetre. It can be mistaken for other Hericium species that grow in the same areas.

The δ-opioid receptor, also known as delta opioid receptor or simply delta receptor, abbreviated DOR or DOP, is an inhibitory 7-transmembrane G-protein coupled receptor coupled to the G protein Gi/G0 and has enkephalins as its endogenous ligands. The regions of the brain where the δ-opioid receptor is largely expressed vary from species model to species model. In humans, the δ-opioid receptor is most heavily expressed in the basal ganglia and neocortical regions of the brain.
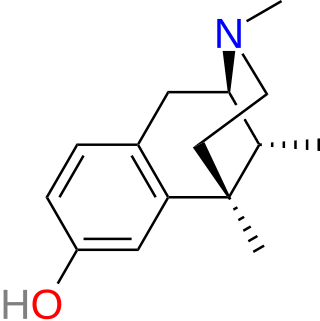
Metazocine is an opioid analgesic related to pentazocine. While metazocine has significant analgesic effects, mediated through a mixed agonist–antagonist action at the mu opioid receptor, its clinical use is limited by dysphoric and hallucinogenic effects which are most likely caused by activity at kappa opioid receptors and/or sigma receptors.

Tifluadom is a benzodiazepine derivative with an unusual activity profile. Unlike most benzodiazepines, tifluadom has no activity at the GABAA receptor, but instead is a selective agonist for the κ-opioid receptor. It has potent analgesic and diuretic effects in animals, and also has sedative effects and stimulates appetite.

SNC-80 is an opioid analgesic compound that selectively activates μ–δ opioid receptor heteromers and is used primarily in scientific research. Discovered in 1994, SNC-80 was a pioneering non-peptide compound regarded as a highly selective agonist for the δ-opioid receptor.

Enadoline is a drug which acts as a highly selective κ-opioid agonist.

Spiradoline (U-62066) is a drug which acts as a highly selective κ-opioid agonist. It has analgesic, diuretic, and antitussive effects, and produces subjective effects in animals similar to those of ketazocine and alazocine. The main effect in humans is sedation, along with analgesic and diuretic effects, but significant side effects such as dysphoria and hallucinations have stopped it from being used clinically.

HZ-2 is a drug which acts as a highly selective κ-opioid agonist. It is a potent analgesic with around the same potency as morphine, with a long duration of action and high oral bioavailability. Side effects include sedation, nausea and dysphoria as well as diuretic effects.
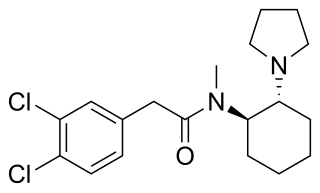
U-50488 is a drug which acts as a highly selective κ-opioid agonist, but without any μ-opioid antagonist effects. It has analgesic, diuretic and antitussive effects, and reverses the memory impairment produced by anticholinergic drugs. U-50488 was one of the first selective kappa agonists invented and research on its derivatives has led to the development of a large family of related compounds. This compound has never received FDA approval and there are no reported human cases in the literature involving an U-50488 overdose.

Hericium is a genus of edible mushrooms in the family Hericiaceae. Species in this genus are white and fleshy and grow on dead or dying wood; fruiting bodies resemble a mass of fragile icicle-like spines that are suspended from either a branched supporting framework or from a tough, unbranched cushion of tissue.
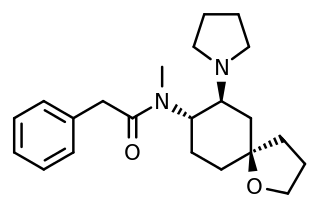
U-69,593 is a drug which acts as a potent and selective κ1-opioid receptor agonist. In animal studies it has been shown to produce antinociception, anti-inflammation, anxiolysis, respiratory depression, and diuresis, while having little effect on gastrointestinal motility. It also inhibits the peripheral, though not central secretion of oxytocin and vasopressin in rats.

Matrine is an alkaloid found in plants from the family Fabaceae. It has a variety of pharmacological effects, including in-vitro anti-cancer effects, as well as κ-opioid and μ-opioid receptor agonism.

Rhodocollybia maculata, commonly known as the spotted toughshank, is a species of basidiomycete fungus in the family Omphalotaceae. It often appears in decomposing conifer duff. R. maculata is a source of collybolide, a sesquiterpenoid containing a furyl-ẟ-lactone motif reminiscent of salvinorin A.
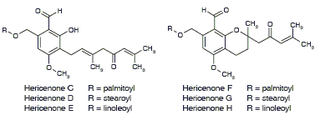
Hericenones is a class of benzaldehydes that are isolates of the fruiting body of Hericium erinaceum that promote nerve growth factor synthesis in vitro.
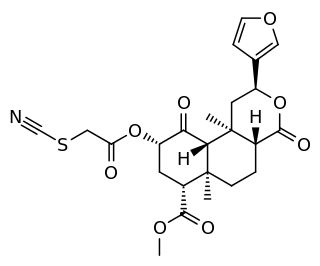
RB-64 is a semi-synthetic derivative of salvinorin A. It is an irreversible agonist, with a reactive thiocyanate group that forms a bond to the κ-opioid receptor (KOR), resulting in very high potency. It is functionally selective, activating G proteins more potently than β-arrestin-2. RB-64 has a bias factor of up to 96 and is analgesic with fewer of the side-effects associated with unbiased KOR agonists. The analgesia is long-lasting. Compared with unbiased agonists, RB-64 evokes considerably less receptor internalization.
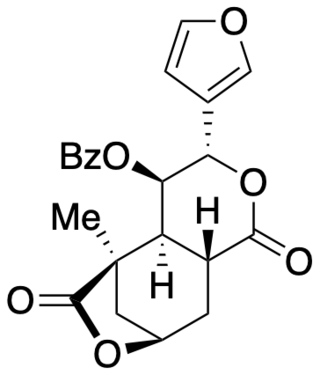
Collybolide is a secondary metabolite of the Rhodocollybia maculata mushroom, a basidiomycete fungus that grows on rotting conifer wood. It was previously believed to be a potent and selective kappa-opioid receptor agonist. However, a total synthesis and independent biological assay determined that collybolide neither excites nor suppresses kappa-opioid receptor signaling. Collybolide is unlikely to be psychoactive, although it has been shown to inhibit L-type calcium channels in isolated rat aorta.