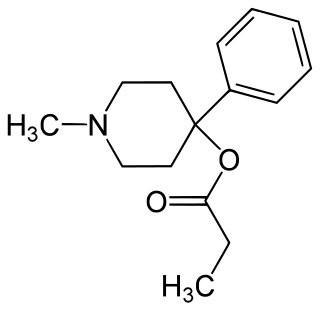
Desmethylprodine or 1-methyl-4-phenyl-4-propionoxypiperidine is an opioid analgesic drug developed in the 1940s by researchers at Hoffmann-La Roche. Desmethylprodine has been labeled by the DEA as a Schedule I drug in the United States. It is an analog of pethidine (meperidine) a Schedule II drug. Chemically, it is a reversed ester of pethidine which has about 70% of the potency of morphine. Unlike its derivative prodine, it does not exhibit optical isomerism. It was reported to have 30 times the activity of pethidine and a greater analgesic effect than morphine in rats, and it was demonstrated to cause central nervous system stimulation in mice.

Pethidine, also known as meperidine and sold under the brand name Demerol among others, is a fully synthetic opioid pain medication of the phenylpiperidine class. Synthesized in 1938 as a potential anticholinergic agent by the German chemist Otto Eisleb, its analgesic properties were first recognized by Otto Schaumann while working for IG Farben, in Germany. Pethidine is the prototype of a large family of analgesics including the pethidine 4-phenylpiperidines, the prodines, bemidones, and others more distant, including diphenoxylate and analogues.
ATC code N02Analgesics is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup N02 is part of the anatomical group N Nervous system.

Phenoperidine, is an opioid analgesic which is structurally related to pethidine and is used clinically as a general anesthetic.
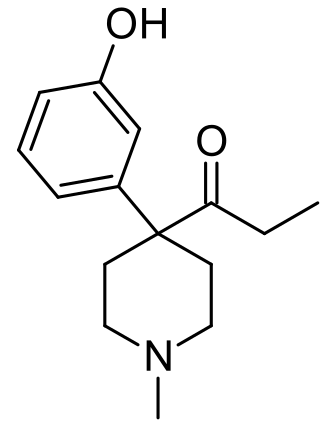
Ketobemidone, sold under the brand name Ketogan among others, is a powerful synthetic opioid painkiller. Its effectiveness against pain is in the same range as morphine, and it also has some NMDA-antagonist properties imparted, in part, by its metabolite norketobemidone. This may make it useful for some types of pain that do not respond well to other opioids. It is marketed in Denmark, Iceland, Norway. Until 2024 it was available in, but is now withdrawn in Sweden. It is used for severe pain.

Hydroxypethidine (Bemidone) is an opioid analgesic that is an analogue of the more commonly used pethidine (meperidine). Hydroxypethidine is slightly more potent than meperidine as an analgesic, 1.5x meperidine in potency, and it also has NMDA antagonist properties like its close relative ketobemidone.

Adrenalone is an adrenergic agonist used as a topical vasoconstrictor and hemostatic. Formerly, it was also used to prolong the action of local anesthetics. It is the ketone form of epinephrine (adrenaline). Contrary to epinephrine, adrenalone mainly acts on alpha-1 adrenergic receptors, but has little affinity for beta receptors. The drug is largely obsolete, being superseded by other hemostatics such as thrombin, fibrinogen, and vasopressin analogues.
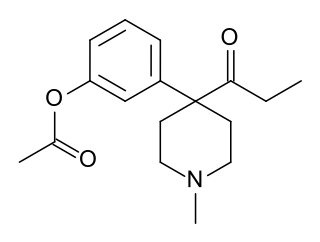
Acetoxyketobemidone (O-Acetylketobemidone) is an opioid analgesic that is an acetylated derivative of ketobemidone. It was developed in the 1950s during research into analogues of pethidine and was assessed by the United Nations Office on Drugs and Crime but was not included on the list of drugs under international control, probably because it was not used in medicine or widely available. Nevertheless, acetoxyketobemidone is controlled as an ester of ketobemidone, which is included in Schedule I of the Single Convention on Narcotic Drugs of 1961.
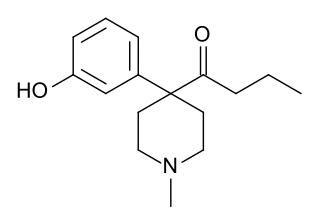
Propylketobemidone is an opioid analgesic that is an analogue of ketobemidone. It was developed in the 1950s during research into analogues of pethidine and was assessed by the United Nations Office on Drugs and Crime but was not included on the list of drugs under international control, probably because it was not used in medicine or widely available.
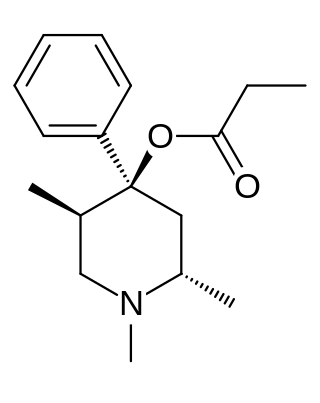
Trimeperidine is an opioid analgesic that is an analogue of prodine. It was developed in the early 1950s in the USSR during research into the related drug pethidine.
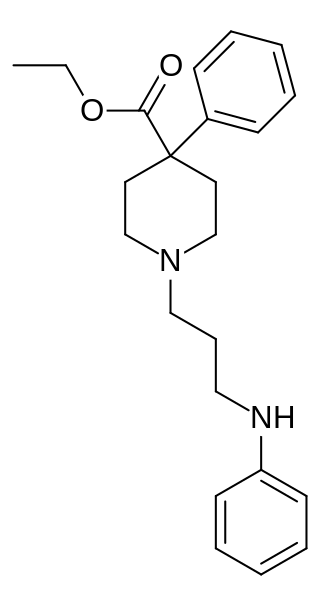
Piminodine (Alvodine) is an opioid analgesic that is an analogue of pethidine (meperidine). It was used in medicine briefly during the 1960s and 70s, but has largely fallen out of clinical use. It was used particularly for obstetric analgesia and in dental procedures and, like pethidine, could be combined with hydroxyzine to intensify the effects. The duration of action is 2–4 hours; 7.5–10 mg via the subcutaneous route is the most common starting dose, being equal to 80–100 mg of pethidine, 40–60 mg of alphaprodine and 10 mg of morphine. Oral formulations were also available.

Norpethidine is a 4-phenylpiperidine derivative that is both a precursor to, and the toxic metabolite of, pethidine (meperidine). It is scheduled by UN Single Convention on Narcotic Drugs. It is a Schedule II Narcotic controlled substance in the United States and has an ACSCN of 9233. The 2014 annual manufacturing quota was 11 grams (0.39 oz).
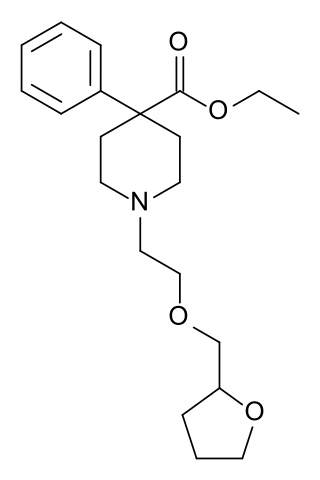
Furethidine is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine), but with around 25x higher potency. According to another source, Furethidine is 500/30 = 16.7 x the potency of pethidine.

Morpheridine (Morpholinoethylnorpethidine) is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine). It is a strong analgesic with around 4 times the potency of pethidine, and unlike pethidine, does not cause convulsions, although it produces the standard opioid side effects such as sedation and respiratory depression.

Pheneridine is a 4-phenylpiperidine derivative that is related to the opioid analgesic drug pethidine (meperidine).

Oxpheneridine is a 4-phenylpiperidine derivative that is related to the opioid analgesic drug pethidine (meperidine).

Pethidine intermediate A is a four-phenylpiperidine derivative that is a precursor to the opioid analgesic drug pethidine (meperidine). It is not known to have any analgesic activity in its own right, however other derivatives of pethidine with a 4-cyano group in place of the carboxylate ethyl ester have been found to be active, so pethidine intermediate A might also show opioid effects. It is scheduled by UN Single Convention on Narcotic Drugs. It is a Schedule II Narcotic controlled substance in the United States and has an ACSCN of 9232. The 2014 annual manufacturing quota was 6 grammes.

The Decree-Law 15/93 of January 22 is a Portuguese drug control law implementing the 1988 United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances.

4-Fluoropethidine is a drug that is a derivative of pethidine (meperidine), which combines pethidine's opioid analgesic effects with increased monoamine reuptake inhibition. It is around 50% less potent than pethidine as an opioid analgesic, but conversely is 50% more potent as a dopamine reuptake inhibitor, with other derivatives such as the 4-iodo and 3,4-dichloro analogues being even more potent dopamine reuptake inhibitors again. However, none of these compounds substitute for cocaine or produce stimulant effects in animals, suggesting that they still act primarily as opioid analgesic drugs in practice. Its action and degree of relation to pethidine means that it may be controlled in those countries which have laws about controlled-substance analogues; it is not itself listed in the Controlled Substances Act 1970.

βk-2C-B (βeta-keto-4-bromo-2,5-dimethoxyphenylamine), also known as bk-2C-B, is a novel psychedelic substance. It is the beta (β) ketone structural analogue of 2C-B, a psychedelic drug of the 2C family. It is used as a recreational drug, usually taken orally. βk-2C-B is a controlled substance in Canada, Germany, Switzerland, and the United Kingdom.



















