A retrovirus is a type of virus that inserts a copy of its RNA genome into the DNA of a host cell that it invades, thus changing the genome of that cell. Once inside the host cell's cytoplasm, the virus uses its own reverse transcriptase enzyme to produce DNA from its RNA genome, the reverse of the usual pattern, thus retro (backwards). The new DNA is then incorporated into the host cell genome by an integrase enzyme, at which point the retroviral DNA is referred to as a provirus. The host cell then treats the viral DNA as part of its own genome, transcribing and translating the viral genes along with the cell's own genes, producing the proteins required to assemble new copies of the virus.

Ribonuclease is a type of nuclease that catalyzes the degradation of RNA into smaller components. Ribonucleases can be divided into endoribonucleases and exoribonucleases, and comprise several sub-classes within the EC 2.7 and 3.1 classes of enzymes.

Ribonuclease H is a family of non-sequence-specific endonuclease enzymes that catalyze the cleavage of RNA in an RNA/DNA substrate via a hydrolytic mechanism. Members of the RNase H family can be found in nearly all organisms, from bacteria to archaea to eukaryotes.

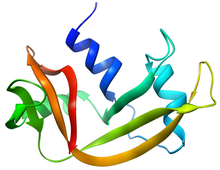
Ranpirnase is a ribonuclease enzyme found in the oocytes of the Northern Leopard Frog. Ranpirnase is a member of the pancreatic ribonuclease protein superfamily and degrades RNA substrates with a sequence preference for uracil and guanine nucleotides. Along with amphinase, another leopard frog ribonuclease, Ranpirnase has been studied as a potential cancer and antiviral treatment due to its unusual mechanism of cytotoxicity tested against transformed cells and antiviral activity.

Ribonuclease L or RNase L, known sometimes as ribonuclease 4 or 2'-5' oligoadenylate synthetase-dependent ribonuclease — is an interferon (IFN)-induced ribonuclease which, upon activation, destroys all RNA within the cell. RNase L is an enzyme that in humans is encoded by the RNASEL gene.

Angiogenin (Ang) also known as ribonuclease 5 is a small 123 amino acid protein that in humans is encoded by the ANG gene. Angiogenin is a potent stimulator of new blood vessels through the process of angiogenesis. Ang hydrolyzes cellular RNA, resulting in modulated levels of protein synthesis and interacts with DNA causing a promoter-like increase in the expression of rRNA. Ang is associated with cancer and neurological disease through angiogenesis and through activating gene expression that suppresses apoptosis.
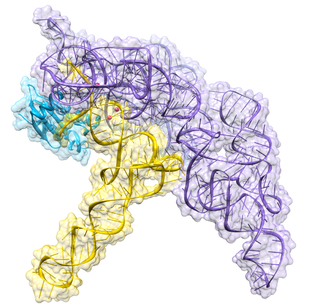
Ribonuclease P is a type of ribonuclease which cleaves RNA. RNase P is unique from other RNases in that it is a ribozyme – a ribonucleic acid that acts as a catalyst in the same way that a protein-based enzyme would. Its function is to cleave off an extra, or precursor, sequence of RNA on tRNA molecules. Further, RNase P is one of two known multiple turnover ribozymes in nature, the discovery of which earned Sidney Altman and Thomas Cech the Nobel Prize in Chemistry in 1989: in the 1970s, Altman discovered the existence of precursor tRNA with flanking sequences and was the first to characterize RNase P and its activity in processing of the 5' leader sequence of precursor tRNA. Recent findings also reveal that RNase P has a new function. It has been shown that human nuclear RNase P is required for the normal and efficient transcription of various small noncoding RNAs, such as tRNA, 5S rRNA, SRP RNA and U6 snRNA genes, which are transcribed by RNA polymerase III, one of three major nuclear RNA polymerases in human cells.

Bovine pancreatic ribonuclease, also often referred to as bovine pancreatic ribonuclease A or simply RNase A, is a pancreatic ribonuclease enzyme that cleaves single-stranded RNA. Bovine pancreatic ribonuclease is one of the classic model systems of protein science. Two Nobel Prizes in Chemistry have been awarded in recognition of work on bovine pancreatic ribonuclease: in 1972, the Prize was awarded to Christian Anfinsen for his work on protein folding and to Stanford Moore and William Stein for their work on the relationship between the protein's structure and its chemical mechanism; in 1984, the Prize was awarded to Robert Bruce Merrifield for development of chemical synthesis of proteins.

Ribonuclease inhibitor (RI) is a large, acidic, leucine-rich repeat protein that forms extremely tight complexes with certain ribonucleases. It is a major cellular protein, comprising ~0.1% of all cellular protein by weight, and appears to play an important role in regulating the lifetime of RNA.
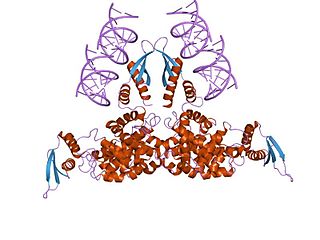
Ribonuclease III (BRENDA 3.1.26.3) is a type of ribonuclease that recognizes dsRNA and cleaves it at specific targeted locations to transform them into mature RNAs. These enzymes are a group of endoribonucleases that are characterized by their ribonuclease domain, which is labelled the RNase III domain. They are ubiquitous compounds in the cell and play a major role in pathways such as RNA precursor synthesis, RNA Silencing, and the pnp autoregulatory mechanism.

Drosha is a Class 2 ribonuclease III enzyme that in humans is encoded by the DROSHA gene.
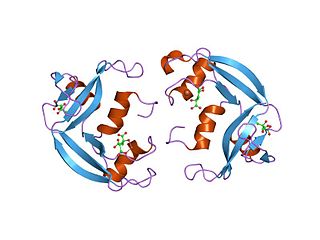
Eosinophil cationic protein (ECP) also known as ribonuclease 3 is a basic protein located in the eosinophil primary matrix. In humans, the eosinophil cationic protein is encoded by the RNASE3 gene.

Eosinophil-derived neurotoxin is an enzyme that in humans is encoded by the RNASE2 gene.

Amphinase is a ribonuclease enzyme found in the oocytes of the Northern leopard frog (Rana pipiens). Amphinase is a member of the pancreatic ribonuclease protein superfamily and degrades long RNA substrates. Along with ranpirnase, another leopard frog ribonuclease, amphinase has been studied as a potential cancer therapy due to its unusual mechanism of cytotoxicity tested against tumor cells.

Ribonuclease pancreatic is an enzyme that in humans is encoded by the RNASE1 gene.

Galectin-10 is an enzyme that in humans is encoded by the CLC gene.

Ribonuclease inhibitor is an enzyme that in humans is encoded by the RNH1 gene.

Ribonuclease 4 is an enzyme that in humans is encoded by the RNASE4 gene.
RNA extraction is the purification of RNA from biological samples. This procedure is complicated by the ubiquitous presence of ribonuclease enzymes in cells and tissues, which can rapidly degrade RNA. Several methods are used in molecular biology to isolate RNA from samples, the most common of these is guanidinium thiocyanate-phenol-chloroform extraction. The filter paper based lysis and elution method features high throughput capacity.
Bovine seminal RNase (BS-RNase) is a member of the ribonuclease superfamily produced by the bovine seminal vesicles. This enzyme can not be differentiated from its members distinctly since there are more features that this enzyme shares with its family members than features that it possess alone. The research on the question of how new functions arrive in proteins in evolution led the scientists to find an uncommon consequence for a usual biological event called gene conversion in the case of the ribonuclease (RNase) protein family. The most well-known member of this family, RNase A, is expressed in the pancreas of oxen. It serves to digest RNA in intestine, and evolved from bacteria fermenting in the stomach of the first ox. The homologous RNase, called seminal RNase, differs from RNase A by 23 amino acids and is expressed in seminal plasma in the concentration of 1-1.5 mg/ml, which constitutes more than 3% of the fluid protein content. Bovine seminal ribonuclease (BS-RNase) is a homologue of RNase A with specific antitumor activity.