An anti-diarrheal drug is any medication which provides symptomatic relief for diarrhea.

Hydromorphone, also known as dihydromorphinone, and sold under the brand name Dilaudid among others, is a morphinan opioid used to treat moderate to severe pain. Typically, long-term use is only recommended for pain due to cancer. It may be used by mouth or by injection into a vein, muscle, or under the skin. Effects generally begin within half an hour and last for up to five hours. A 2016 Cochrane review found little difference in benefit between hydromorphone and other opioids for cancer pain.

Pethidine, also known as meperidine and sold under the brand name Demerol among others, is a fully synthetic opioid pain medication of the phenylpiperidine class. Synthesized in 1938 as a potential anticholinergic agent by the German chemist Otto Eisleb, its analgesic properties were first recognized by Otto Schaumann while working for IG Farben, in Germany. Pethidine is the prototype of a large family of analgesics including the pethidine 4-phenylpiperidines, the prodines, bemidones, and others more distant, including diphenoxylate and analogues.

Carisoprodol, sold under the brand name Soma among others, is a medication used for musculoskeletal pain. Effects generally begin within half an hour and last for up to six hours. It is taken orally.
Carfentanil or carfentanyl, sold under the brand name Wildnil, is an extremely potent opioid analgesic used in veterinary medicine to anesthetize large animals such as elephants and rhinoceroses. It is an analogue of fentanyl, of which it is structurally derivative. It is typically administered in this context by tranquilizer dart. Carfentanil has also been used in humans to image opioid receptors. It has additionally been used as a recreational drug, typically by injection, insufflation, or inhalation. Deaths have been reported in association with carfentanil.

Levorphanol is an opioid medication used to treat moderate to severe pain. It is the levorotatory enantiomer of the compound racemorphan. Its dextrorotatory counterpart is dextrorphan.

Mequitazine (trade name Primalan) is an H1 antagonist and anticholinergic of the phenothiazine chemical class. It is used to treat allergies and rhinitis.
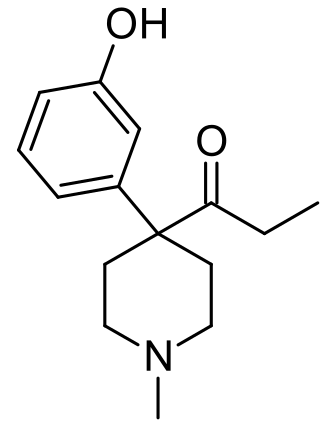
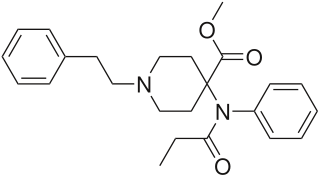
Ketobemidone, sold under the brand name Ketogan among others, is a powerful synthetic opioid painkiller. Its effectiveness against pain is in the same range as morphine, and it also has some NMDA-antagonist properties imparted, in part, by its metabolite norketobemidone. This may make it useful for some types of pain that do not respond well to other opioids. It is marketed in Denmark, Iceland, Norway. Until 2024 it was available in, but is now withdrawn in Sweden. It is used for severe pain.

Hydroxypethidine (Bemidone) is an opioid analgesic that is an analogue of the more commonly used pethidine (meperidine). Hydroxypethidine is slightly more potent than meperidine as an analgesic, 1.5x meperidine in potency, and it also has NMDA antagonist properties like its close relative ketobemidone.

Dihydroetorphine was developed by K. W. Bentley at McFarlan-Smith in the 1960s and is a potent opioid analgesic used mainly in China. It is a derivative of the better-known opioid etorphine, a very potent veterinary painkiller and anesthetic medication used primarily for the sedation of large animals such as elephants, giraffes, and rhinos.

Nalorphine is a mixed opioid agonist–antagonist with opioid antagonist and analgesic properties. It was introduced in 1954 and was used as an antidote to reverse opioid overdose and in a challenge test to determine opioid dependence.

Lofentanil or lofentanyl is one of the most potent opioid analgesics known and is an analogue of fentanyl, which was developed in 1960. It is most similar to the highly potent opioid carfentanil (4-carbomethoxyfentanyl), only slightly more potent. Lofentanil can be described as 3-methylcarfentanil, or 3-methyl-4-carbomethoxyfentanyl. While 3-methylfentanyl is considerably more potent than fentanyl itself, lofentanil is only slightly stronger than carfentanil. This suggests that substitution at both the 3 and 4 positions of the piperidine ring introduces steric hindrance which prevents μ-opioid affinity from increasing much further. As with other 3-substituted fentanyl derivatives such as ohmefentanyl, the stereoisomerism of lofentanil is very important, with some stereoisomers being much more potent than others.

Adrenalone is an adrenergic agonist used as a topical vasoconstrictor and hemostatic. Formerly, it was also used to prolong the action of local anesthetics. It is the ketone form of epinephrine (adrenaline). Contrary to epinephrine, adrenalone mainly acts on alpha-1 adrenergic receptors, but has little affinity for beta receptors. The drug is largely obsolete, being superseded by other hemostatics such as thrombin, fibrinogen, and vasopressin analogues.
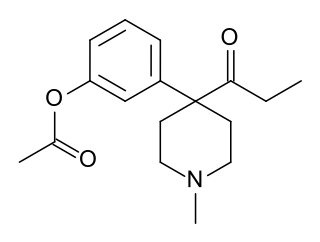
Acetoxyketobemidone (O-Acetylketobemidone) is an opioid analgesic that is an acetylated derivative of ketobemidone. It was developed in the 1950s during research into analogues of pethidine and was assessed by the United Nations Office on Drugs and Crime but was not included on the list of drugs under international control, probably because it was not used in medicine or widely available. Nevertheless, acetoxyketobemidone is controlled as an ester of ketobemidone, which is included in Schedule I of the Single Convention on Narcotic Drugs of 1961.
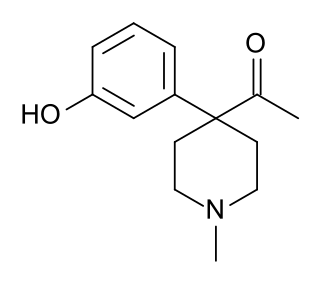
Methylketobemidone is an opioid analgesic that is an analogue of ketobemidone. It was developed in the 1950s during research into analogues of pethidine and was assessed by the United Nations Office on Drugs and Crime but was not included on the list of drugs under international control, probably because it was not used in medicine or widely available.

Picenadol (LY-97435) is a 4-phenylpiperidine derivative that is an opioid analgesic drug developed by Eli Lilly in the 1970s.
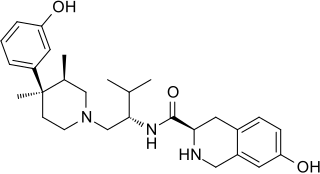
JDTic is a selective, long-acting ("inactivating") antagonist of the κ-opioid receptor (KOR). JDTic is a 4-phenylpiperidine derivative, distantly related structurally to analgesics such as pethidine and ketobemidone, and more closely to the MOR antagonist alvimopan. In addition, it is structurally distinct from other KOR antagonists such as norbinaltorphimine. JDTic has been used to create crystal structures of KOR [ PDB: 4DJH, 6VI4].

3-Methoxyphencyclidine (3-MeO-PCP) is a dissociative hallucinogen of the arylcyclohexylamine class related to phencyclidine (PCP) which has been sold online as a designer drug. It has been used across Europe and the United States. In some cases, consumption has been known to be fatal. It acts mainly as an NMDA receptor antagonist, though it has also been found to interact with the sigma σ1 receptor and the serotonin transporter. The drug does not possess any opioid activity nor does it act as a dopamine reuptake inhibitor.

βk-2C-B (βeta-keto-4-bromo-2,5-dimethoxyphenylamine), also known as bk-2C-B, is a novel psychedelic substance. It is the beta (β) ketone structural analogue of 2C-B, a psychedelic drug of the 2C family. It is used as a recreational drug, usually taken orally. βk-2C-B is a controlled substance in Canada, Germany, Switzerland, and the United Kingdom.

Acetoxymethylketobemidone (O-AMKD), is an opioid designer drug related to ketobemidone, with around the same potency as morphine. It was first identified in Germany in October 2020.



















