Related Research Articles

Elapidae is a family of snakes characterized by their permanently erect fangs at the front of the mouth. Most elapids are venomous, with the exception of the genus Emydocephalus. Many members of this family exhibit a threat display of rearing upwards while spreading out a neck flap. Elapids are endemic to tropical and subtropical regions around the world, with terrestrial forms in Asia, Australia, Africa, and the Americas and marine forms in the Pacific and Indian Oceans. Members of the family have a wide range of sizes, from the 18 cm (7.1 in) white-lipped snake to the 5.85 m king cobra. Most species have neurotoxic venom that is channeled by their hollow fangs, and some may contain other toxic components in varying proportions. The family includes 55 genera with around 360 species and over 170 subspecies.

Snake venom is a highly toxic saliva containing zootoxins that facilitates in the immobilization and digestion of prey. This also provides defense against threats. Snake venom is usually injected by unique fangs during a bite, though some species are also able to spit venom.

The enzyme phospholipase A2 (EC 3.1.1.4, PLA2, systematic name phosphatidylcholine 2-acylhydrolase) catalyses the cleavage of fatty acids in position 2 of phospholipids, hydrolyzing the bond between the second fatty acid "tail" and the glycerol molecule:

The inland taipan, also commonly known as the western taipan, small-scaled snake, or fierce snake, is a species of extremely venomous snake in the family Elapidae. The species is endemic to semiarid regions of central east Australia. Aboriginal Australians living in those regions named the snake dandarabilla. It was formally described by Frederick McCoy in 1879 and then by William John Macleay in 1882, but for the next 90 years, it was a mystery to the scientific community; no further specimens were found, and virtually nothing was added to the knowledge of this species until its rediscovery in 1972.

Crotalus scutulatus is known commonly as the Mohave Rattlesnake. Other common English names include Mojave Rattlesnake and, referring specifically to the nominate (northern) subspecies: Northern Mohave Rattlesnake and Mojave Green Rattlesnake, the latter name commonly shortened to the more colloquial “Mojave green”. Campbell and Lamar (2004) supported the English name “Mohave (Mojave) rattlesnake” with some reluctance because so little of the snake’s range lies within the Mojave Desert.

Delta atracotoxin is a low-molecular-weight neurotoxic polypeptide found in the venom of the Sydney funnel-web spider.
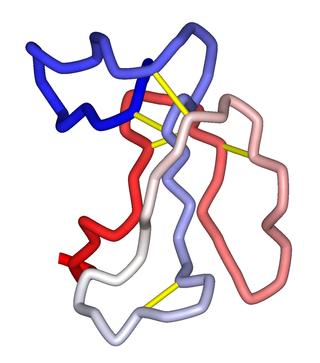
α-Bungarotoxin is one of the bungarotoxins, components of the venom of the elapid Taiwanese banded krait snake. It is a type of α-neurotoxin, a neurotoxic protein that is known to bind competitively and in a relatively irreversible manner to the nicotinic acetylcholine receptor found at the neuromuscular junction, causing paralysis, respiratory failure, and death in the victim. It has also been shown to play an antagonistic role in the binding of the α7 nicotinic acetylcholine receptor in the brain, and as such has numerous applications in neuroscience research.
Taicatoxin (TCX) is a snake toxin that blocks voltage-dependent L-type calcium channels and small conductance Ca2+-activated K+ channels. The name taicatoxin (TAIpan + CAlcium + TOXIN) is derived from its natural source, the taipan snake, the site of its action, calcium channels, and from its function as a toxin. Taicatoxin was isolated from the venom of Australian taipan snake, Oxyuranus scutellatus scutellatus. TCX is a secreted protein, produced in the venom gland of the snake.
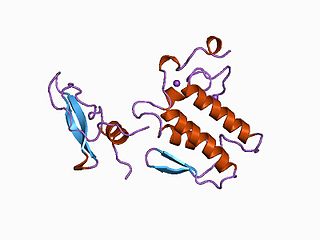
β-Bungarotoxin is a form of bungarotoxin that is fairly common in Krait venoms. It is the prototypic class of snake β-neurotoxins. There are at least five isoforms, coded β1 to β5, assembled from different combinations of A and Bchains.
Bestoxin is a neurotoxin from the venom of the South African spitting scorpion Parabuthus transvaalicus. Most likely, it targets sodium channel function, thus promoting spontaneous and repetitive neuronal firing. Following injection into mice, it causes non-lethal writhing behaviour.
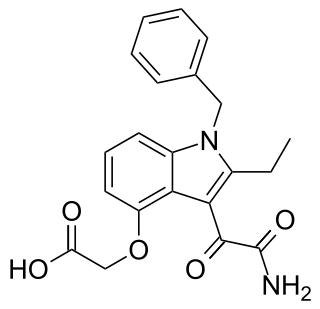
Varespladib is an inhibitor of the IIa, V, and X isoforms of secretory phospholipase A2 (sPLA2). The molecule acts as an anti-inflammatory agent by disrupting the first step of the arachidonic acid pathway of inflammation. From 2006 to 2012, varespladib was under active investigation by Anthera Pharmaceuticals as a potential therapy for several inflammatory diseases, including acute coronary syndrome and acute chest syndrome. The trial was halted in March 2012 due to inadequate efficacy. The selective sPLA2 inhibitor varespladib (IC50 value 0.009 μM in chromogenic assay, mole fraction 7.3X10-6) was studied in the VISTA-16 randomized clinical trial (clinicaltrials.gov Identifier: NCT01130246) and the results were published in 2014. The sPLA2 inhibition by varespladib in this setting seemed to be potentially harmful, and thus not a useful strategy for reducing adverse cardiovascular outcomes from acute coronary syndrome. Since 2016, scientific research has focused on the use of Varespladib as an inhibitor of snake venom toxins using various types of in vitro and in vivo models. Varespladib showed a significant inhibitory effect to snake venom PLA2 which makes it a potential first-line drug candidate in snakebite envenomation therapy. In 2019, the U.S. Food and Drug Administration (FDA) granted varespladib orphan drug status for its potential to treat snakebite.

α-Neurotoxins are a group of neurotoxic peptides found in the venom of snakes in the families Elapidae and Hydrophiidae. They can cause paralysis, respiratory failure, and death. Members of the three-finger toxin protein family, they are antagonists of post-synaptic nicotinic acetylcholine receptors (nAChRs) in the neuromuscular synapse that bind competitively and irreversibly, preventing synaptic acetylcholine (ACh) from opening the ion channel. Over 100 α-neurotoxins have been identified and sequenced.
The Papuan black snake is a highly venomous snake of the family Elapidae native to New Guinea. Reaching around 2 m in length, it is a predominantly black snake coloured grey underneath.

κ-Bungarotoxin is a protein neurotoxin of the bungarotoxin family that is found in the venom of the many-banded krait, a snake found in Taiwan. κ-Bungarotoxin is a high affinity antagonist of nicotinic acetylcholine receptors (nAChRs), particularly of CHRNA3; it causes a post-synaptic blockade of neurotransmission. Although there is significant variability in the clinical effects of snake bites, neuromuscular paralysis and respiratory failure are associated with krait bites.

Three-finger toxins are a protein superfamily of small toxin proteins found in the venom of snakes. Three-finger toxins are in turn members of a larger superfamily of three-finger protein domains which includes non-toxic proteins that share a similar protein fold. The group is named for its common structure consisting of three beta strand loops connected to a central core containing four conserved disulfide bonds. The 3FP protein domain has no enzymatic activity and is typically between 60-74 amino acid residues long. Despite their conserved structure, three-finger toxin proteins have a wide range of pharmacological effects. Most members of the family are neurotoxins that act on cholinergic intercellular signaling; the alpha-neurotoxin family interacts with muscle nicotinic acetylcholine receptors (nAChRs), the kappa-bungarotoxin family with neuronal nAChRs, and muscarinic toxins with muscarinic acetylcholine receptors (mAChRs).
Crotoxin (CTX) is the main toxic compound in the snake venom of the South American rattlesnake, Crotalus durissus terrificus. Crotoxin is a heterodimeric beta-neurotoxin, composed of an acidic, non-toxic and non-enzymatic subunit (CA), and a basic, weakly toxic, phospholipase A2 protein (CB). This neurotoxin causes paralysis by both pre- and postsynaptic blocking of acetylcholine signalling.
Venomics is the large-scale study of proteins associated with venom. Venom is a toxic substance secreted by animals, which is typically injected either offensively or defensively into prey or aggressors, respectively.
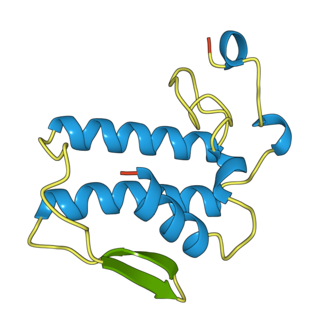
Notexin is a toxin produced by the tiger snake (Notechis scutatus). It is a myotoxic and presynaptic, neurotoxic phospholipase A2 (PLA2s). These are enzymes that hydrolyze the bond between a fatty acid tail and glycerol in fatty acids on the 2-position.
MiDCA1, short for Micrurus dumerili carinicauda 1, is a β-neurotoxin primarily affecting presynaptic synapses, where it interferes with the release of neurotransmitters by inhibiting potassium (K+) channels. This toxin belongs to the phospholipase A2 (PLA2) family but distinguishes itself by existing as a monomer, unlike some other PLA2 toxins. It occurs naturally in the venom of the coral snake Micrurus dumerili carinicauda.
References
- 1 2 3 4 5 Fohlman J, Eaker D, Karlsoon E, Thesleff S (September 1976). "Taipoxin, an extremely potent presynaptic neurotoxin from the venom of the australian snake taipan (Oxyuranus s. scutellatus). Isolation, characterization, quaternary structure and pharmacological properties". European Journal of Biochemistry. 68 (2): 457–69. doi: 10.1111/j.1432-1033.1976.tb10833.x . PMID 976268.
- ↑ Silva A, Hodgson WC, Isbister GK (October 2016). "Cross-Neutralisation of In Vitro Neurotoxicity of Asian and Australian Snake Neurotoxins and Venoms by Different Antivenoms". Toxins. 8 (10): 302. doi: 10.3390/toxins8100302 . PMC 5086662 . PMID 27763543.
- ↑ Alomone labs: Taipoxin (pdf)
- ↑ Rossetto O, Morbiato L, Caccin P, Rigoni M, Montecucco C (June 2006). "Presynaptic enzymatic neurotoxins". Journal of Neurochemistry. 97 (6): 1534–45. doi: 10.1111/j.1471-4159.2006.03965.x . PMID 16805767. S2CID 22678139.
- ↑ Davidson FF, Dennis EA (September 1990). "Evolutionary relationships and implications for the regulation of phospholipase A2 from snake venom to human secreted forms". Journal of Molecular Evolution. 31 (3): 228–38. Bibcode:1990JMolE..31..228D. doi:10.1007/BF02109500. PMID 2120459. S2CID 6203372.
- 1 2 3 Montecucco C, Rossetto O (June 2008). "On the quaternary structure of taipoxin and textilotoxin: the advantage of being multiple". Toxicon. 51 (8): 1560–2. doi:10.1016/j.toxicon.2008.03.020. PMID 18471843.
- 1 2 3 Alape-Girón A, Persson B, Cederlund E, Flores-Díaz M, Gutiérrez JM, Thelestam M, et al. (January 1999). "Elapid venom toxins: multiple recruitments of ancient scaffolds". European Journal of Biochemistry. 259 (1–2): 225–34. doi: 10.1046/j.1432-1327.1999.00021.x . PMID 9914497. S2CID 2136068.
- ↑ Kini RM (1997). Venom Phospholipase A2 Enzymes. Chichester: Wiley. ISBN 978-0471961895.
- ↑ Fletcher JE, Jiang MS (December 1995). "Presynaptically acting snake venom phospholipase A2 enzymes attack unique substrates". Toxicon. 33 (12): 1565–76. doi:10.1016/0041-0101(95)00108-5. PMID 8866614.
- ↑ Fohlman J, Lind P, Eaker D (December 1977). "Taipoxin, an extremely potent presynaptic snake venom neurotoxin. Elucidation of the primary structure of the acidic carbohydrate-containing taipoxin-subunit, a prophospholipase homolog". FEBS Letters. 84 (2): 367–71. doi: 10.1016/0014-5793(77)80726-6 . PMID 563806.
- ↑ Lomonte B, Tarkowski A, Hanson LA (November 1994). "Broad cytolytic specificity of myotoxin II, a lysine-49 phospholipase A2 of Bothrops asper snake venom". Toxicon. 32 (11): 1359–69. doi:10.1016/0041-0101(94)90408-1. PMID 7886694.
- ↑ Gutiérrez JM, Lomonte B (November 1995). "Phospholipase A2 myotoxins from Bothrops snake venoms". Toxicon. 33 (11): 1405–24. doi:10.1016/0041-0101(95)00085-z. hdl: 10669/29394 . PMID 8744981.
- 1 2 "Taipan Antivenom". www.csl.com.au. Retrieved 2017-03-17.
- ↑ Hyatt MC, Russell JA (October 1981). "Effects of beta-bungarotoxin and taipoxin on contractions of canine airways caused by nerve stimulation". Life Sciences. 29 (17): 1755–9. doi:10.1016/0024-3205(81)90185-5. PMID 7300571.
- 1 2 Harris JB, Maltin CA (May 1982). "Myotoxic activity of the crude venom and the principal neurotoxin, taipoxin, of the Australian taipan, Oxyuranus scutellatus". British Journal of Pharmacology. 76 (1): 61–75. doi:10.1111/j.1476-5381.1982.tb09191.x. PMC 2068749 . PMID 7082907.
- ↑ Harris JB, MacDonell CA (1981-01-01). "Phospholipase A2 activity of notexin and its role in muscle damage". Toxicon. 19 (3): 419–30. doi:10.1016/0041-0101(81)90046-5. PMID 7245222.
- ↑ Dixon RW, Harris JB (February 1999). "Nerve terminal damage by beta-bungarotoxin: its clinical significance". The American Journal of Pathology. 154 (2): 447–55. doi:10.1016/S0002-9440(10)65291-1. PMC 1850016 . PMID 10027403.
- ↑ Harris JB, Grubb BD, Maltin CA, Dixon R (February 2000). "The neurotoxicity of the venom phospholipases A(2), notexin and taipoxin". Experimental Neurology. 161 (2): 517–26. doi:10.1006/exnr.1999.7275. PMID 10686073. S2CID 6714210.
- ↑ Neco P, Rossetto O, Gil A, Montecucco C, Gutiérrez LM (April 2003). "Taipoxin induces F-actin fragmentation and enhances release of catecholamines in bovine chromaffin cells". Journal of Neurochemistry. 85 (2): 329–37. doi:10.1046/j.1471-4159.2003.01682.x. PMID 12675909. S2CID 8907229.
- ↑ Kirkpatrick LL, Matzuk MM, Dodds DC, Perin MS (June 2000). "Biochemical interactions of the neuronal pentraxins. Neuronal pentraxin (NP) receptor binds to taipoxin and taipoxin-associated calcium-binding protein 49 via NP1 and NP2". The Journal of Biological Chemistry. 275 (23): 17786–92. doi: 10.1074/jbc.M002254200 . PMID 10748068.
- ↑ Dodds DC, Omeis IA, Cushman SJ, Helms JA, Perin MS (August 1997). "Neuronal pentraxin receptor, a novel putative integral membrane pentraxin that interacts with neuronal pentraxin 1 and 2 and taipoxin-associated calcium-binding protein 49". The Journal of Biological Chemistry. 272 (34): 21488–94. doi: 10.1074/jbc.272.34.21488 . PMID 9261167.
- ↑ Leslie CC, Gelb MH (2004). "Assaying Phospholipase A2 Activity". Signal Transduction Protocols. Methods in Molecular Biology. Vol. 284. Methods Mol. Biol. pp. 229–42. doi:10.1385/1-59259-816-1:229. ISBN 1-59259-816-1. PMID 15173620.
- ↑ Rigoni M, Caccin P, Gschmeissner S, Koster G, Postle AD, Rossetto O, et al. (December 2005). "Equivalent effects of snake PLA2 neurotoxins and lysophospholipid-fatty acid mixtures". Science. 310 (5754): 1678–80. Bibcode:2005Sci...310.1678R. CiteSeerX 10.1.1.817.8280 . doi:10.1126/science.1120640. JSTOR 3842969. PMID 16339444. S2CID 39970648.
- ↑ Caccin P, Rigoni M, Bisceglie A, Rossetto O, Montecucco C (November 2006). "Reversible skeletal neuromuscular paralysis induced by different lysophospholipids". FEBS Letters. 580 (27): 6317–21. doi: 10.1016/j.febslet.2006.10.039 . PMID 17083939. S2CID 38178998.
- ↑ Megighian A, Rigoni M, Caccin P, Zordan MA, Montecucco C (April 2007). "A lysolecithin/fatty acid mixture promotes and then blocks neurotransmitter release at the Drosophila melanogaster larval neuromuscular junction". Neuroscience Letters. 416 (1): 6–11. doi:10.1016/j.neulet.2007.01.040. PMID 17293048. S2CID 7635663.
- ↑ Kuruppu S, Chaisakul J, Smith AI, Hodgson WC (April 2014). "Inhibition of presynaptic neurotoxins in taipan venom by suramin". Neurotoxicity Research. 25 (3): 305–10. doi:10.1007/s12640-013-9426-z. PMID 24129771. S2CID 16083544.