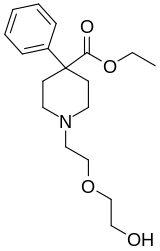
Alfentanil is a potent but short-acting synthetic opioid analgesic drug, used for anaesthesia in surgery. It is an analogue of fentanyl with around one-fourth to one-tenth the potency, one-third the duration of action, and an onset of action four times faster than that of fentanyl. Alfentanil has a pKa of approximately 6.5, which leads to a very high proportion of the drug being uncharged at physiologic pH, a characteristic responsible for its rapid onset. It is an agonist at mu opioid receptors.
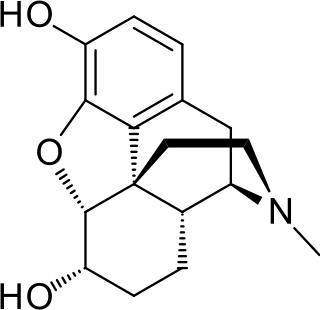
Dihydromorphine is a semi-synthetic opioid structurally related to and derived from morphine. The 7,8-double bond in morphine is reduced to a single bond to get dihydromorphine. Dihydromorphine is a moderately strong analgesic and is used clinically in the treatment of pain and also is an active metabolite of the analgesic opioid drug dihydrocodeine. Dihydromorphine occurs in trace quantities in assays of opium on occasion, as does dihydrocodeine, dihydrothebaine, tetrahydrothebaine, etc. The process for manufacturing dihydromorphine from morphine for pharmaceutical use was developed in Germany in the late 19th century, with the synthesis being published in 1900 and the drug introduced clinically as Paramorfan shortly thereafter. A high-yield synthesis from tetrahydrothebaine was later developed.

Dextromoramide is a powerful opioid analgesic approximately three times more potent than morphine but shorter acting. It is subject to drug prohibition regimes, both internationally through UN treaties and by the criminal law of individual nations, and is usually prescribed only in the Netherlands.

Butorphanol is a morphinan-type synthetic agonist–antagonist opioid analgesic developed by Bristol-Myers. Butorphanol is most closely structurally related to levorphanol. Butorphanol is available as the tartrate salt in injectable, tablet, and intranasal spray formulations. The tablet form is only used in dogs, cats and horses due to low bioavailability in humans.

Methorphan comes in two isomeric forms, each with differing pharmacology and effects:

Lefetamine (Santenol) is a drug which is a stimulant and also an analgesic with effects comparable to codeine.

Piritramide(R-3365, trade names Dipidolor, Piridolan, Pirium and others) is a synthetic opioid analgesic that is marketed in certain European countries including: Austria, Belgium, Czech Republic, Slovenia, Germany and the Netherlands. It comes in free form, is about 0.75x times as potent as morphine and is given parenterally for the treatment of severe pain. Nausea, vomiting, respiratory depression and constipation are believed to be less frequent with piritramide than with morphine, and it produces more rapid-onset analgesia when compared to morphine and pethidine. After intravenous administration the onset of analgesia is as little as 1–2 minutes, which may be related to its great lipophilicity. The analgesic and sedative effects of piritramide are believed to be potentiated with phenothiazines and its emetic (nausea/vomiting-inducing) effects are suppressed. The volume of distribution is 0.7-1 L/kg after a single dose, 4.7-6 L/kg after steady-state concentrations are achieved and up to 11.1 L/kg after prolonged dosing.
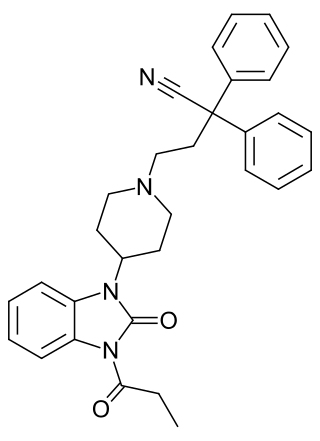
Bezitramide is an opioid analgesic. Bezitramide itself is a prodrug which is readily hydrolyzed in the gastrointestinal tract to its active metabolite, despropionyl-bezitramide. Bezitramide was discovered at Janssen Pharmaceutica in 1961. It is most commonly marketed under the trade name Burgodin.

Benzylmorphine (Peronine) is a semi-synthetic opioid narcotic introduced to the international market in 1896 and that of the United States very shortly thereafter. It is much like codeine, containing a benzyl group attached to the morphine molecule just as the methyl group creates codeine and the ethyl group creates ethylmorphine or dionine. It is about 90% as strong as codeine by weight.
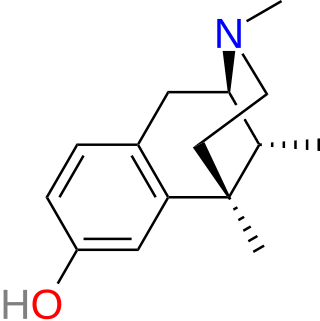
Metazocine is an opioid analgesic related to pentazocine. While metazocine has significant analgesic effects, mediated through a mixed agonist–antagonist action at the mu opioid receptor, its clinical use is limited by dysphoric and hallucinogenic effects which are most likely caused by activity at kappa opioid receptors and/or sigma receptors.
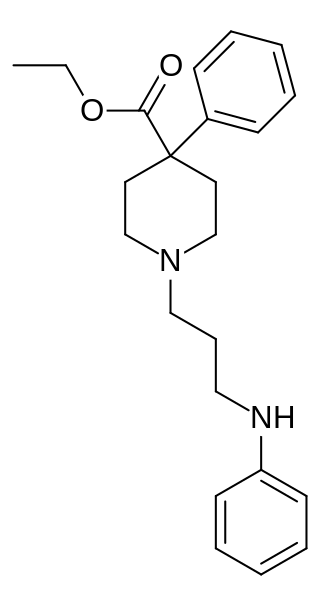
Piminodine (Alvodine) is an opioid analgesic that is an analogue of pethidine (meperidine). It was used in medicine briefly during the 1960s and 70s, but has largely fallen out of clinical use. It was used particularly for obstetric analgesia and in dental procedures and, like pethidine, could be combined with hydroxyzine to intensify the effects. The duration of action is 2–4 hours; 7.5–10 mg via the subcutaneous route is the most common starting dose, being equal to 80–100 mg of pethidine, 40–60 mg of alphaprodine and 10 mg of morphine. Oral formulations were also available.

Phenazocine is an opioid analgesic drug, which is related to pentazocine and has a similar profile of effects.

Benzethidine is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine.
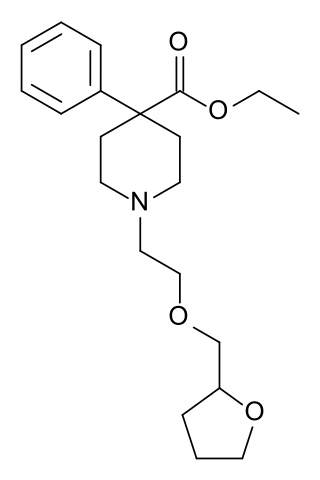
Furethidine is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine), but with around 25x higher potency. According to another source, Furethidine is 500/30 = 16.7 x the potency of pethidine.

Morpheridine (Morpholinoethylnorpethidine) is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine). It is a strong analgesic with around 4 times the potency of pethidine, and unlike pethidine, does not cause convulsions, although it produces the standard opioid side effects such as sedation and respiratory depression.

Acetylmethadol, also known as methadyl acetate, is a synthetic opioid analgesic. It is a racemic mixture of alphacetylmethadol (α-acetylmethadol) and betacetylmethadol (β-acetylmethadol), which are in turn racemic mixtures of levacetylmethadol and D-α-acetylmethadol and L-β-acetylmethadol and D-β-acetylmethadol, respectively. Hence, acetylmethadol has four possible optical isomers. All of these isomers have been shown to partially or fully substitute for the discriminative stimulus effects of heroin in rats, and thus it can be inferred that, in addition to LAAM which is used clinically as such, they are all likely to be active opioid analgesics in humans.

Alphamethadol (INN), or α-methadol, also known as alfametadol, is a synthetic opioid analgesic. It is an isomer of dimepheptanol (methadol), the other being betamethadol (β-methadol). Alphamethadol is composed of two isomers itself, L-α-methadol, and D-α-methadol. The former compound, L-α-methadol, is an important active metabolite of levacetylmethadol (LAAM), an opioid substitute drug that is used clinically. Both of alphamethadol's isomers bind to and activate the μ-opioid receptor and are active as opioid analgesics, similarly to those of alphacetylmethadol (α-acetylmethadol).

Levophenacylmorphan is a morphinan derivative that acts as an opioid agonist. It has potent analgesic effects and is around 10x more potent than morphine. Adverse effects associated with its use are those of the opioids as a whole, including pruritus, nausea, respiratory depression, euphoria and development of tolerance and dependence to its effects.

Isomethadone (INN, BAN; trade name Liden; also known as isoamidone) is a synthetic opioid analgesic and antitussive related to methadone that was used formerly as a pharmaceutical drug but is now no longer marketed. Isomethadone was used as both an analgesic and antitussive. It binds to and activates both the μ- and δ-opioid receptors, with the (S)-isomer being the more potent of the two enantiomers. Isomethadone is a Schedule II controlled substance in the United States, with an ACSCN of 9226 and a 2014 aggregate manufacturing quota of 5 g. The salts in use are the hydrobromide (HBr, free base conversion ratio 0.793), hydrochloride (HCl, 0.894), and HCl monohydrate (0.850). Isomethadone is also regulated internationally as a Schedule I controlled substance under the United Nations Single Convention on Narcotic Drugs of 1961.

Noracymethadol (INN) is a synthetic opioid analgesic related to methadone that was never marketed. In a clinical trial of postpartum patients it was reported to produce analgesia comparable to that of morphine but with less nausea, dizziness, and drowsiness. Other side effects included salivation, ataxia, and respiratory depression that was reversible by naloxone. Similarly to many of its analogues, noracymethadol is a Schedule I controlled substance in the United States with an ACSCN of 9633 and 2013 annual manufacturing quota of 12 grammes. and is also controlled internationally under the United Nations Single Convention on Narcotic Drugs of 1961. The salts known are the gluconate and hydrochloride (0.903).