Related Research Articles

3,4-Methyl

Empathogens or entactogens are a class of psychoactive drugs that induce the production of experiences of emotional communion, oneness, relatedness, emotional openness—that is, empathy or sympathy—as particularly observed and reported for experiences with 3,4-methylenedioxymethamphetamine (MDMA). This class of drug is distinguished from the classes of hallucinogen or psychedelic, and amphetamine or stimulants. Major members of this class include MDMA, MDA, MDEA, MDOH, MBDB, 5-APB, 5-MAPB, 6-APB, 6-MAPB, methylone, mephedrone, GHB, αMT, and αET, MDAI among others. Most entactogens are phenethylamines and amphetamines, although several, such as αMT and αET, are tryptamines. When referring to MDMA and its counterparts, the term MDxx is often used. Entactogens are sometimes incorrectly referred to as hallucinogens or stimulants, although many entactogens such as ecstasy exhibit psychedelic or stimulant properties as well.
Psychedelic therapy refers to the proposed use of psychedelic drugs, such as psilocybin, ayahuasca, LSD, psilocin, mescaline (peyote), DMT, 5-MeO-DMT, Ibogaine, MDMA, to treat mental disorders. As of 2021, psychedelic drugs are controlled substances in most countries and psychedelic therapy is not legally available outside clinical trials, with some exceptions.
The Multidisciplinary Association for Psychedelic Studies (MAPS) is an American nonprofit organization working to raise awareness and understanding of psychedelic substances. MAPS was founded in 1986 by Rick Doblin and is now based in San Jose, California.
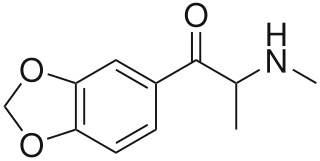
Methylone, also known as 3,4-methylenedioxy-N-methylcathinone (MDMC), is an entactogen and stimulant drug of the amphetamine, cathinone, and benzodioxole families related to 3,4-methylenedioxymethamphetamine. It is the β-keto or cathinone analogue of MDMA. Methylone is usually taken orally, but is also used by other routes.
Neurocrine Biosciences, Inc. is an American biopharmaceutical company founded in 1992. It is headquartered in San Diego, California, and led by CEO Kevin Gorman. Neurocrine develops treatments for neurological and endocrine-related diseases and disorders. In 2017, the company's drug valbenazine (Ingrezza) was approved in the US to treat adults with tardive dyskinesia (TD).

The substituted methylenedioxyphenethylamines represent a diverse chemical class of compounds derived from phenethylamines. This category encompasses numerous psychoactive substances with entactogenic, psychedelic, and/or stimulant properties, in addition to entheogens. These compounds find application as research chemicals, designer drugs, and recreational substances.
Substituted amphetamines, or simply amphetamines, are a class of compounds based upon the amphetamine structure; it includes all derivative compounds which are formed by replacing, or substituting, one or more hydrogen atoms in the amphetamine core structure with substituents. The compounds in this class span a variety of pharmacological subclasses, including stimulants, empathogens, and hallucinogens, among others. Examples of substituted amphetamines are amphetamine (itself), methamphetamine, ephedrine, cathinone, phentermine, mephentermine, tranylcypromine, bupropion, methoxyphenamine, selegiline, amfepramone (diethylpropion), pyrovalerone, MDMA (ecstasy), and DOM (STP).

Mind Medicine (MindMed) Inc., doing business as MindMed, is a New York-based biotechnology company that is currently developing clinical and therapeutic applications for psychedelic and, more broadly, psychoplastogenic drugs.
MYCO-005 is a serotonin 5-HT2A receptor agonist and psychedelic hallucinogen which is under development for the treatment of depressive disorders and anxiety disorders.
Matthew John Baggott, PhD is an American neuroscientist who studies entactogens, hallucinogens, and other psychoactive drugs. He is considered to be an expert on MDMA and other entactogens and is an influential figure in the psychedelic medicine movement.
Tactogen is a public benefit corporation and start-up pharmaceutical company based in Palo Alto, California that is developing novel MDMA-like entactogens and psychedelics as medicines. Its stated goal is to develop new MDMA-like drugs with improved effectiveness, tolerability, and safety, as well as gentleness and accessibility, for treatment of psychiatric disorders and other conditions. Tactogen was co-founded by neuroscientist Matthew J. Baggott and Luke Pustejovsky in 2020. Baggott is the chief executive officer (CEO) while Pustejovsky is the chief operating officer (COO).

The substituted benzothiophenes are a class of chemical compounds based on benzothiophene. They are closely related to the substituted benzofurans, substituted tryptamines, and to other chemical groups such as the substituted benzodioxoles.
Compass Pathways, or COMPASS Pathways, is a British pharmaceutical company developing psychedelics as potential medicines. Its main drug candidate, psilocybin (COMP360), is currently in phase 3 clinical trials for treatment-resistant depression. This candidate has received breakthrough therapy status from the U.S. Food and Drug Administration (FDA). It is the most advanced psychedelic drug candidate in development besides Lykos Therapeutics's midomafetamine (MDMA).
Mydecine Innovations Group, or simply Mydecine, is an American and Canadian pharmaceutical company that is developing psychedelics and entactogens as medicines.
MYCO-006 is an MDMA-like entactogen that is under development for the treatment of psychiatric disorders. It is a short-acting and fast-onset MDMA analogue. Based on animal studies, it is predicted that MYCO-006 will have a duration of 1 to 2 hours, about one-third the 6- to 8-hour duration of MDMA, and to onset 4 times as fast as MDMA. MYCO-006 is being developed by Mydecine. As of February 2024, it is in preclinical research. The chemical structure of MYCO-006 does not yet appear to have been disclosed. However, MYCO-006 is said to be a benzodioxole like MDMA. 5-BZT-MDMA (MY100) and 6-BZT-MDMA (MY101) were described in Mydecine's patent for short-acting MDMA analogues.
MYCO-007 is an MDMA-like entactogen that is under development for the treatment of psychiatric disorders. It is a short-acting MDMA analogue. MYCO-007 is being developed by Mydecine. As of February 2024, it is in preclinical research. The chemical structure of MYCO-007 does not yet appear to have been disclosed. 5-BZT-MDMA (MY100) and 6-BZT-MDMA (MY101) were described in Mydecine's patent for short-acting MDMA analogues
MYCO-004 is a patch-delivered psychedelic tryptamine which is under development for the treatment of psychiatric disorders. It is anticipated to allow for precision dosing and to have a short duration of approximately 2 hours. The drug is being developed by Mydecine. As of December 2021, it is in preclinical research for psychiatric disorders. The exact chemical structure of MYCO-004 does not yet seem to have been disclosed.
Cybin IRL is a Canadian pharmaceutical company that is developing psychedelic drugs as medicines.
References
- 1 2 3 4 5 "MYCO 002". AdisInsight. 28 November 2021. Retrieved 29 January 2025.
MYCO 002 is an entactogenic MDMA-like compound being developed by Mydecine Innovations for the potential treatment of mental disorders. [...]
- 1 2 3 4 "Delving into the Latest Updates on MYCO-002 with Synapse". Synapse. 23 January 2025. Retrieved 29 January 2025.
- 1 2 Mydecine Innovations Group (7 April 2021). "Mydecine Announces Four Lead Novel Drug Candidates and Prepares for Pre-IND Meetings with the FDA and Health Canada To Prepare For Human Clinical". GlobeNewswire News Room. Retrieved 29 January 2025.
The four initial drug candidates include: [...] MYCO - 002 is an entactogenic compound that has been created with the goal of reducing harm and improving the safety profile vs. traditional MDMA.