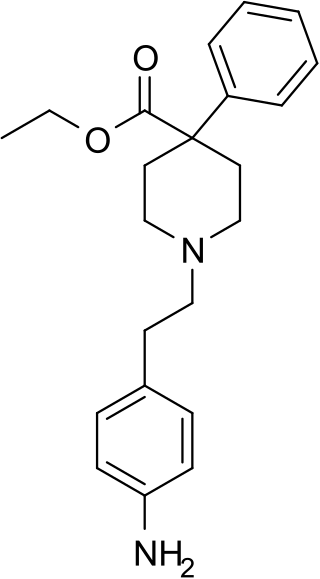
Anileridine is a synthetic analgesic drug and is a member of the piperidine class of analgesic agents developed by Merck & Co. in the 1950s. It differs from pethidine (meperidine) in that the N-methyl group of meperidine is replaced by an N-aminophenethyl group, which increases its analgesic activity.

Methorphan comes in two isomeric forms, each with differing pharmacology and effects:

Phenoperidine, is an opioid analgesic which is structurally related to pethidine and is used clinically as a general anesthetic.

Piritramide(R-3365, trade names Dipidolor, Piridolan, Pirium and others) is a synthetic opioid analgesic that is marketed in certain European countries including: Austria, Belgium, Czech Republic, Slovenia, Germany and the Netherlands. It comes in free form, is about 0.75x times as potent as morphine and is given parenterally for the treatment of severe pain. Nausea, vomiting, respiratory depression and constipation are believed to be less frequent with piritramide than with morphine, and it produces more rapid-onset analgesia when compared to morphine and pethidine. After intravenous administration the onset of analgesia is as little as 1–2 minutes, which may be related to its great lipophilicity. The analgesic and sedative effects of piritramide are believed to be potentiated with phenothiazines and its emetic (nausea/vomiting-inducing) effects are suppressed. The volume of distribution is 0.7-1 L/kg after a single dose, 4.7-6 L/kg after steady-state concentrations are achieved and up to 11.1 L/kg after prolonged dosing.

Hydroxypethidine (Bemidone) is an opioid analgesic that is an analogue of the more commonly used pethidine (meperidine). Hydroxypethidine is slightly more potent than meperidine as an analgesic, 1.5x meperidine in potency, and it also has NMDA antagonist properties like its close relative ketobemidone.

Allylprodine is an opioid analgesic that is an analog of prodine. It was discovered by Hoffman-La Roche in 1957 during research into the related drug pethidine. Derivatives were tested to prove the theory that phenolic and non-phenolic opioids bind at different sites of the opiate receptor.
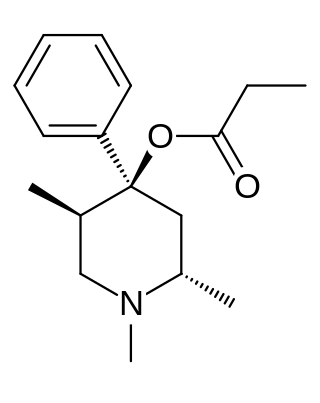
Trimeperidine is an opioid analgesic that is an analogue of prodine. It was developed in the early 1950s in the USSR during research into the related drug pethidine.

Meprodine is an opioid analgesic that is an analogue of pethidine (meperidine). It is closely related to the drug prodine, the only difference being that meprodine has an ethyl group rather than a methyl at the 3-position of the piperidine ring.
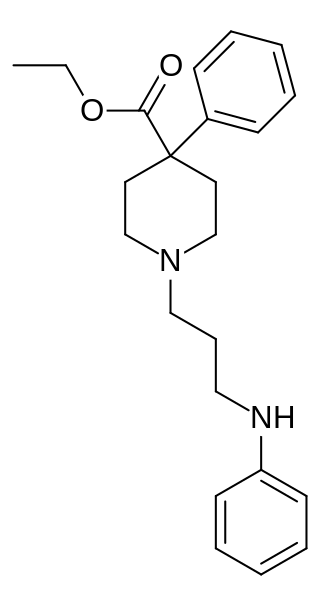
Piminodine (Alvodine) is an opioid analgesic that is an analogue of pethidine (meperidine). It was used in medicine briefly during the 1960s and 70s, but has largely fallen out of clinical use. It was used particularly for obstetric analgesia and in dental procedures and, like pethidine, could be combined with hydroxyzine to intensify the effects. The duration of action is 2–4 hours; 7.5–10 mg via the subcutaneous route is the most common starting dose, being equal to 80–100 mg of pethidine, 40–60 mg of alphaprodine and 10 mg of morphine. Oral formulations were also available.

Propiram is a partial μ-opioid receptor agonist and weak μ antagonist analgesic from the ampromide family of drugs related to other drugs such as phenampromide and diampromide. It was invented in 1963 in the United Kingdom by Bayer but was not widely marketed, although it saw some limited clinical use, especially in dentistry. Propiram reached Phase III clinical trials in the United States and Canada.

Diampromide is an opioid analgesic from the ampromide family of drugs, related to other drugs such as propiram and phenampromide. It was invented in the 1960s by American Cyanamid, and can be described as a ring-opened analogue of fentanyl.

Proheptazine is an opioid analgesic related to pethidine. It was invented in the 1960s.

Benzethidine is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine.

Etoxeridine is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine).
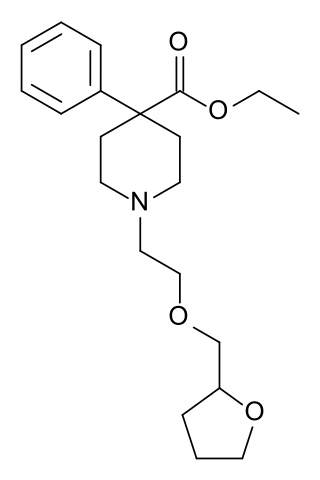
Furethidine is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine), but with around 25x higher potency. According to another source, Furethidine is 500/30 = 16.7 x the potency of pethidine.

Pethidine intermediate A is a four-phenylpiperidine derivative that is a precursor to the opioid analgesic drug pethidine (meperidine). It is not known to have any analgesic activity in its own right, however other derivatives of pethidine with a 4-cyano group in place of the carboxylate ethyl ester have been found to be active, so pethidine intermediate A might also show opioid effects. It is scheduled by UN Single Convention on Narcotic Drugs. It is a Schedule II Narcotic controlled substance in the United States and has an ACSCN of 9232. The 2014 annual manufacturing quota was 6 grammes.

Drotebanol (Oxymethebanol) is a morphinan derivative that acts as an opioid agonist. It was invented by Sankyo Company in Japan during the 1970s. It is synthesised from thebaine.

Racemorphan, or morphanol, is the racemic mixture of the two stereoisomers of 17-methylmorphinan-3-ol, each with differing pharmacology and effects:

Moramide intermediate is a moramide precursor scheduled by UN Single Convention on Narcotic Drugs.

Methadone intermediate is a methadone precursor scheduled by UN Single Convention on Narcotic Drugs. It is a Schedule II Narcotic controlled substance in the United States and has an ACSCN of 9254. The 2014 annual manufacturing quota was 32 875 kilos. It is listed as a Schedule I drug in Canada, but is only significant as a precursor for methadone, as it does not have analgesic activity in its own right, though it does show some atropine-like activity.




















