| Thermus | |
|---|---|
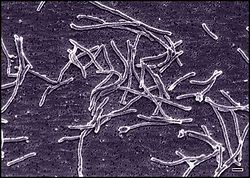 | |
| Thermus aquaticus | |
| Scientific classification | |
| Domain: | Bacteria |
| Kingdom: | Thermotogati |
| Phylum: | Deinococcota |
| Class: | Deinococci |
| Order: | Thermales |
| Family: | Thermaceae |
| Genus: | Thermus Brock & Freeze 1969 |
| Type species | |
| Thermus aquaticus Brock & Freeze 1969 | |
| Species | |
Thermus is a genus of thermophilic bacteria. It is one of several bacteria belonging to the Deinococcota phylum. According to comparative analysis of 16S rRNA, this is one of the most ancient group of bacteria. [1] Thermus species can be distinguished from other genera in the family Thermaceae as well as all other bacteria by the presence of eight conserved signature indels found in proteins such as adenylate kinase and replicative DNA helicase as well as 14 conserved signature proteins that are exclusively shared by members of this genus. [2]