In chemistry, azide is a linear, polyatomic anion with the formula N−3 and structure −N=N+=N−. It is the conjugate base of hydrazoic acid HN3. Organic azides are organic compounds with the formula RN3, containing the azide functional group. The dominant application of azides is as a propellant in air bags.
In chemistry, a nitride is a chemical compound of nitrogen. Nitrides can be inorganic or organic, ionic or covalent. The nitride anion, N3- ion, is very elusive but compounds of nitride are numerous, although rarely naturally occurring. Some nitrides have a found applications, such as wear-resistant coatings (e.g., titanium nitride, TiN), hard ceramic materials (e.g., silicon nitride, Si3N4), and semiconductors (e.g., gallium nitride, GaN). The development of GaN-based light emitting diodes was recognized by the 2014 Nobel Prize in Physics. Metal nitrido complexes are also common.
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorganic molecules of the general forms Ps–Ps or Ps–X, such as cyanogen; pseudohalide anions, such as cyanide ion; inorganic acids, such as hydrogen cyanide; as ligands in coordination complexes, such as ferricyanide; and as functional groups in organic molecules, such as the nitrile group. Well-known pseudohalogen functional groups include cyanide, cyanate, thiocyanate, and azide.
Pentazole is an aromatic molecule consisting of a five-membered ring with all nitrogen atoms, one of which is bonded to a hydrogen atom. It has the molecular formula HN5. Although strictly speaking a homocyclic, inorganic compound, pentazole has historically been classed as the last in a series of heterocyclic azole compounds containing one to five nitrogen atoms. This set contains pyrrole, imidazole, pyrazole, triazoles, tetrazole, and pentazole.

In coordination chemistry, hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated. In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity, and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands.

Phenyl azide is an organic compound with the formula C6H5N3. It is one of the prototypical organic azides. It is a pale yellow oily liquid with a pungent odor. The structure consists of a linear azide substituent bound to a phenyl group. The C−N=N angle is approximately 120°. It was discovered in 1864 by Peter Griess by the reaction of ammonia and phenyldiazonium.

Gold compounds are compounds by the element gold (Au). Although gold is the most noble of the noble metals, it still forms many diverse compounds. The oxidation state of gold in its compounds ranges from −1 to +5, but Au(I) and Au(III) dominate its chemistry. Au(I), referred to as the aurous ion, is the most common oxidation state with soft ligands such as thioethers, thiolates, and organophosphines. Au(I) compounds are typically linear. A good example is Au(CN)−2, which is the soluble form of gold encountered in mining. The binary gold halides, such as AuCl, form zigzag polymeric chains, again featuring linear coordination at Au. Most drugs based on gold are Au(I) derivatives.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.
Tetramethylammonium pentafluoroxenate is a chemical compound with the chemical formula [N(CH3)4]+[XeF5]−. This salt consists of tetramethylammonium cations [N(CH3)4]+ and pentafluoroxenate(IV) anions [XeF5]−. The [XeF5]− ion was the first example of a pentagonal planar molecular geometry AX5E2 species. It was prepared by the reaction of [N(CH3)4]F with xenon tetrafluoride, [N(CH3)4]F being chosen because it can be prepared in anhydrous form and is readily soluble in organic solvents. The anion is planar, with the fluorine atoms in a slightly distorted pentagonal coordination. Other salts have been prepared with sodium, caesium and rubidium, and vibrational spectra show that these contain the same planar ion. The isolated anion has the point group of D5h.
Dinitrogen difluoride is a chemical compound with the formula N2F2. It is a gas at room temperature, and was first identified in 1952 as the thermal decomposition product of the fluorine azide. It has the structure F−N=N−F and exists in both cis and trans isomers, as typical for diimides.

In chemistry, the pentazenium cation is a positively-charged polyatomic ion with the chemical formula N+5 and structure N−N−N−N−N. Together with solid nitrogen polymers and the azide anion, it is one of only three poly-nitrogen species obtained in bulk quantities.
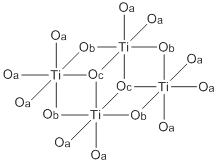
Titanium ethoxide is a chemical compound with the formula Ti4(OCH2CH3)16. It is a commercially available colorless liquid that is soluble in organic solvents but hydrolyzes readily. Its structure is more complex than suggested by its empirical formula. Like other alkoxides of titanium(IV) and zirconium(IV), it finds used in organic synthesis and materials science.
Phosphinoimidates, also known as phophinimides, are the anions derived from phosphine imides with the structure [R3P=N]− (R = alkyl or aryl). Phosphinimide ligands are used to for transition metal complexes that are highly active catalysts in some olefin polymerization reactions.

Karl Otto Christe is an inorganic chemist. He is the best reference in respectful handling of a huge number of extremely reactive components and his extensive experience in fluorine chemistry earned him the title of 'The Fluorine God'. His research covers fluorine chemistry of nitrogen and halogens and the synthesis of new energetic materials.

Nitrosyl cyanide, a blue-green gas, is the compound with the molecular formula ONCN. The compound has been invoked as a product of the oxidation of cyanamide catalyzed by the enzyme glucose oxidase.

Transition metal azide complexes are coordination complexes containing one or more azide (N3−) ligands. In addition to coordination complexes, this article summarizes homoleptic transition metal azides, which are often coordination polymers.

Pentazenium tetraazidoborate is an extremely unstable chemical compound with the formula N5[B(N3)4]. It is a white solid that violently explodes at room temperature. This compound has a 95.7% nitrogen content which is the second highest known of a chemical compound, exceeding even that of ammonium azide (93.3%) and 1-diazidocarbamoyl-5-azidotetrazole (89.1%), being surpassed only by hydrazoic acid (97.7%).
Main group azido compounds are chemical compounds consisting of azide, N3- bonded to a main group element.
Tellurium tetraazide is an inorganic chemical compound with the formula Te(N3)4. It is a highly sensitive explosive and takes the form of a yellow solid. It has been prepared directly as a precipitate of the reaction between tellurium tetrafluoride and trimethylsilyl azide.
Selenium tetraazide is an inorganic chemical compound with the formula Se(N3)4. It is a highly sensitive explosive, and has been prepared directly from selenium tetrafluoride and trimethylsilyl azide.









