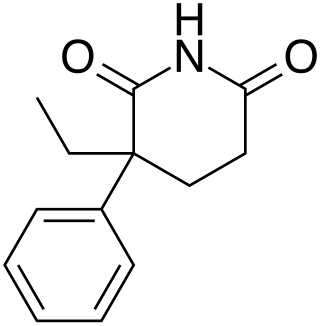
Glutethimide is a hypnotic sedative that was introduced by Ciba in 1954 as a safe alternative to barbiturates to treat insomnia. Before long, however, it had become clear that glutethimide was just as likely to cause addiction and caused similar withdrawal symptoms. Doriden was the brand-name version. Current production levels in the United States point to its use only in small-scale research. Manufacturing of the drug was discontinued in the US in 1993 and discontinued in several eastern European countries in 2006.
The Convention for Limiting the Manufacture and Regulating the Distribution of Narcotic Drugs was a drug control treaty promulgated in Geneva on 13 July 1931 that entered into force on 9 July 1933.

Ethylmorphine is an opioid analgesic and antitussive.
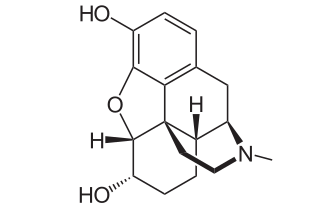
Dihydromorphine is a semi-synthetic opioid structurally related to and derived from morphine. The 7,8-double bond in morphine is reduced to a single bond to get dihydromorphine. Dihydromorphine is a moderately strong analgesic and is used clinically in the treatment of pain and also is an active metabolite of the analgesic opioid drug dihydrocodeine. Dihydromorphine occurs in trace quantities in assays of opium on occasion, as does dihydrocodeine, dihydrothebaine, tetrahydrothebaine, etc. The process for manufacturing dihydromorphine from morphine for pharmaceutical use was developed in Germany in the late 19th century, with the synthesis being published in 1900 and the drug introduced clinically as Paramorfan shortly thereafter. A high-yield synthesis from tetrahydrothebaine was later developed.

Levomethorphan (LVM) (INN, BAN) is an opioid analgesic of the morphinan family that has never been marketed. It is the L-stereoisomer of racemethorphan (methorphan). The effects of the two isomers of racemethorphan are quite different, with dextromethorphan (DXM) being an antitussive at low doses and a dissociative hallucinogen at much higher doses. Levomethorphan is about five times stronger than morphine.

Lefetamine (Santenol) is a drug which is a stimulant and also an analgesic with effects comparable to codeine.

Thebacon, or dihydrocodeinone enol acetate, is a semisynthetic opioid that is similar to hydrocodone and is most commonly synthesised from thebaine. Thebacon was invented in Germany in 1924, four years after the first synthesis of hydrocodone. Thebacon is a derivative of acetyldihydrocodeine, where only the 6–7 double bond is saturated. Thebacon is marketed as its hydrochloride salt under the trade name Acedicon, and as its bitartrate under Diacodin and other trade names. The hydrochloride salt has a free base conversion ratio of 0.846. Other salts used in research and other settings include thebacon's phosphate, hydrobromide, citrate, hydroiodide, and sulfate.

Nicocodeine is an opioid analgesic and cough suppressant, an ester of codeine closely related to dihydrocodeine and the codeine analogue of nicomorphine. It is not commonly used in most countries, but has activity similar to other opiates. Nicocodeine and nicomorphine were synthesized in 1904, and introduced in 1957 by Lannacher Heilmittel of Austria. Nicocodeine is metabolised in the liver by demethylation to produce nicomorphine, also known as 6-nicotinoylmorphine, and subsequently further metabolised to morphine. Side effects are similar to those of other opiates and include itching, nausea and respiratory depression. Related opioid analogues such as nicomorphine and nicodicodeine were first synthesized. The definitive synthesis, which involves treating anhydrous codeine base with nicotinic anhydride at 130 °C, was published by Pongratz and Zirm in Monatshefte für Chemie in 1957, simultaneously with the two analogues in an article about amides and esters of various organic acids.

Acetyldihydrocodeine is an opiate derivative discovered in Germany in 1914 and was used as a cough suppressant and analgesic. It is not commonly used, but has activity similar to other opiates. Acetyldihydrocodeine is a very close relative derivative of thebacon, where only the 6-7 bond is unsaturated. Acetyldihydrocodeine can be described as the 6-acetyl derivative of dihydrocodeine and is metabolised in the liver by demethylation and deacetylation to produce dihydromorphine.

Benzylmorphine (Peronine) is a semi-synthetic opioid narcotic introduced to the international market in 1896 and that of the United States very shortly thereafter. It is much like codeine, containing a benzyl group attached to the morphine molecule just as the methyl group creates codeine and the ethyl group creates ethylmorphine or dionine. It is about 90% as strong as codeine by weight.

Nalorphine is a mixed opioid agonist–antagonist with opioid antagonist and analgesic properties. It was introduced in 1954 and was used as an antidote to reverse opioid overdose and in a challenge test to determine opioid dependence.
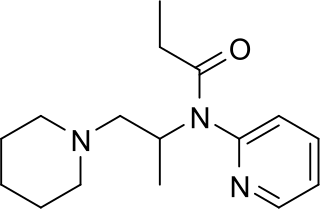
Propiram is a partial μ-opioid receptor agonist and weak μ antagonist analgesic from the ampromide family of drugs related to other drugs such as phenampromide and diampromide. It was invented in 1963 in the United Kingdom by Bayer but was not widely marketed, although it saw some limited clinical use, especially in dentistry. Propiram reached Phase III clinical trials in the United States and Canada.

Phenampromide is an opioid analgesic from the ampromide family of drugs, related to other drugs such as propiram and diampromide. It was invented in the 1960s by American Cyanamid Co. Although never given a general release, it was research found that 60 mg of phenampromide is equivalent to about 50 mg of codeine. Tests on its two enantiomers showed that all of the analgesic effects were caused by the (S)-isomer. Introduction of a phenyl group to the 4-position of the piperidine-ring produces a drug 60-fold more potent than morphine. The most potent reported derivative is 4-hydroxy-4-phenyl phenapromide which displays analgesic activity some x150 greater than morphine.

Myrophine (Myristylbenzylmorphine) is an opiate analogue that was developed in 1952. It is a derivative of morphine.

Drotebanol (Oxymethebanol) is a morphinan derivative that acts as an opioid agonist. It was invented by Sankyo Company in Japan during the 1970s. It is synthesised from thebaine.
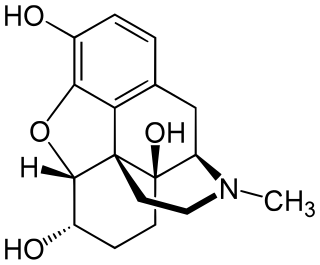
Hydromorphinol, is an opiate analogue that is a derivative of morphine, where the 14-position has been hydroxylated and the 7,8- double bond saturated. It has similar effects to morphine such as sedation, analgesia and respiratory depression, but is twice as potent as morphine and has a steeper dose-response curve and longer half-life. It is used in medicine as the bitartrate salt and hydrochloride

Normorphine is an opiate analogue, the N-demethylated derivative of morphine, that was first described in the 1950s when a large group of N-substituted morphine analogues were characterized for activity. The compound has relatively little opioid activity in its own right, but is a useful intermediate which can be used to produce both opioid antagonists such as nalorphine, and also potent opioid agonists such as N-phenethylnormorphine. with its formation from morphine catalyzed by the liver enzymes CYP3A4 and CYP2C8.
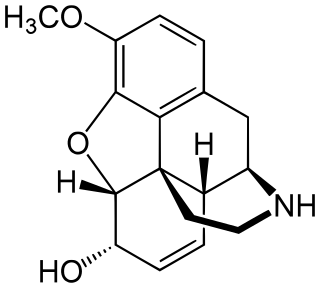
Norcodeine is an opiate analogue that is the N-demethylated derivative of codeine. It has relatively little opioid activity in its own right, but is formed as a metabolite of codeine following ingestion.

Racemorphan, or morphanol, is the racemic mixture of the two stereoisomers of 17-methylmorphinan-3-ol, each with differing pharmacology and effects:

Morphine-N-oxide (genomorphine) is an active opioid metabolite of morphine. Morphine itself, in trials with rats, is 11–22 times more potent than morphine-N-oxide subcutaneously and 39–89 times more potent intraperitoneally. However, pretreatment with amiphenazole or tacrine increases the potency of morphine-N-oxide in relation to morphine. A possible explanation is that morphine-N-oxide is rapidly inactivated in the liver and impairment of inactivation processes or enzymes increases functionality.



















