Within a nervous system, a neuron, neurone, or nerve cell is an electrically excitable cell that fires electric signals called action potentials across a neural network. Neurons communicate with other cells via synapses, which are specialized connections that commonly use minute amounts of chemical neurotransmitters to pass the electric signal from the presynaptic neuron to the target cell through the synaptic gap.

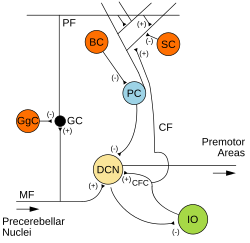
The cerebellum is a major feature of the hindbrain of all vertebrates. Although usually smaller than the cerebrum, in some animals such as the mormyrid fishes it may be as large as it or even larger. In humans, the cerebellum plays an important role in motor control and cognitive functions such as attention and language as well as emotional control such as regulating fear and pleasure responses, but its movement-related functions are the most solidly established. The human cerebellum does not initiate movement, but contributes to coordination, precision, and accurate timing: it receives input from sensory systems of the spinal cord and from other parts of the brain, and integrates these inputs to fine-tune motor activity. Cerebellar damage produces disorders in fine movement, equilibrium, posture, and motor learning in humans.

The olfactory bulb is a neural structure of the vertebrate forebrain involved in olfaction, the sense of smell. It sends olfactory information to be further processed in the amygdala, the orbitofrontal cortex (OFC) and the hippocampus where it plays a role in emotion, memory and learning. The bulb is divided into two distinct structures: the main olfactory bulb and the accessory olfactory bulb. The main olfactory bulb connects to the amygdala via the piriform cortex of the primary olfactory cortex and directly projects from the main olfactory bulb to specific amygdala areas. The accessory olfactory bulb resides on the dorsal-posterior region of the main olfactory bulb and forms a parallel pathway. Destruction of the olfactory bulb results in ipsilateral anosmia, while irritative lesions of the uncus can result in olfactory and gustatory hallucinations.
An inhibitory postsynaptic potential (IPSP) is a kind of synaptic potential that makes a postsynaptic neuron less likely to generate an action potential. The opposite of an inhibitory postsynaptic potential is an excitatory postsynaptic potential (EPSP), which is a synaptic potential that makes a postsynaptic neuron more likely to generate an action potential. IPSPs can take place at all chemical synapses, which use the secretion of neurotransmitters to create cell-to-cell signalling. EPSPs and IPSPs compete with each other at numerous synapses of a neuron. This determines whether an action potential occurring at the presynaptic terminal produces an action potential at the postsynaptic membrane. Some common neurotransmitters involved in IPSPs are GABA and glycine.

Pyramidal cells, or pyramidal neurons, are a type of multipolar neuron found in areas of the brain including the cerebral cortex, the hippocampus, and the amygdala. Pyramidal cells are the primary excitation units of the mammalian prefrontal cortex and the corticospinal tract. One of the main structural features of the pyramidal neuron is the conic shaped soma, or cell body, after which the neuron is named. Other key structural features of the pyramidal cell are a single axon, a large apical dendrite, multiple basal dendrites, and the presence of dendritic spines.

Basket cells are inhibitory GABAergic interneurons of the brain, found throughout different regions of the cortex and cerebellum.
An apical dendrite is a dendrite that emerges from the apex of a pyramidal cell. Apical dendrites are one of two primary categories of dendrites, and they distinguish the pyramidal cells from spiny stellate cells in the cortices. Pyramidal cells are found in the prefrontal cortex, the hippocampus, the entorhinal cortex, the olfactory cortex, and other areas. Dendrite arbors formed by apical dendrites are the means by which synaptic inputs into a cell are integrated. The apical dendrites in these regions contribute significantly to memory, learning, and sensory associations by modulating the excitatory and inhibitory signals received by the pyramidal cells.

Neurotransmission is the process by which signaling molecules called neurotransmitters are released by the axon terminal of a neuron, and bind to and react with the receptors on the dendrites of another neuron a short distance away. A similar process occurs in retrograde neurotransmission, where the dendrites of the postsynaptic neuron release retrograde neurotransmitters that signal through receptors that are located on the axon terminal of the presynaptic neuron, mainly at GABAergic and glutamatergic synapses.
Depolarization-induced suppression of inhibition is the classical and original electrophysiological example of endocannabinoid function in the central nervous system. Prior to the demonstration that depolarization-induced suppression of inhibition was dependent on the cannabinoid CB1 receptor function, there was no way of producing an in vitro endocannabinoid mediated effect.
The stratum lucidum of the hippocampus is a layer of the hippocampus between the stratum pyramidale and the stratum radiatum. It is the tract of the mossy fiber projections, both inhibitory and excitatory from the granule cells of the dentate gyrus. One mossy fiber may make up to 37 connections to a single pyramidal cell, and innervate around 12 pyramidal cells on top of that. Any given pyramidal cell in the stratum lucidum may get input from as many as 50 granule cells.

In the hippocampus, the mossy fiber pathway consists of unmyelinated axons projecting from granule cells in the dentate gyrus that terminate on modulatory hilar mossy cells and in Cornu Ammonis area 3 (CA3), a region involved in encoding short-term memory. These axons were first described as mossy fibers by Santiago Ramón y Cajal as they displayed varicosities along their lengths that gave them a mossy appearance. The axons that make up the pathway emerge from the basal portions of the granule cells and pass through the hilus of the dentate gyrus before entering the stratum lucidum of CA3. Granule cell synapses tend to be glutamatergic, though immunohistological data has indicated that some synapses contain neuropeptidergic elements including opiate peptides such as dynorphin and enkephalin. There is also evidence for co-localization of both GABAergic and glutamatergic neurotransmitters within mossy fiber terminals. GABAergic and glutamatergic co-localization in mossy fiber boutons has been observed primarily in the developing hippocampus, but in adulthood, evidence suggests that mossy fiber synapses may alternate which neurotransmitter is released through activity-dependent regulation.

Hippocampus anatomy describes the physical aspects and properties of the hippocampus, a neural structure in the medial temporal lobe of the brain. It has a distinctive, curved shape that has been likened to the sea-horse monster of Greek mythology and the ram's horns of Amun in Egyptian mythology. This general layout holds across the full range of mammalian species, from hedgehog to human, although the details vary. For example, in the rat, the two hippocampi look similar to a pair of bananas, joined at the stems. In primate brains, including humans, the portion of the hippocampus near the base of the temporal lobe is much broader than the part at the top. Due to the three-dimensional curvature of this structure, two-dimensional sections such as shown are commonly seen. Neuroimaging pictures can show a number of different shapes, depending on the angle and location of the cut.

The anatomy of the cerebellum can be viewed at three levels. At the level of gross anatomy, the cerebellum consists of a tightly folded and crumpled layer of cortex, with white matter underneath, several deep nuclei embedded in the white matter, and a fluid-filled ventricle in the middle. At the intermediate level, the cerebellum and its auxiliary structures can be broken down into several hundred or thousand independently functioning modules or compartments known as microzones. At the microscopic level, each module consists of the same small set of neuronal elements, laid out with a highly stereotyped geometry.

Cerebellar granule cells form the thick granular layer of the cerebellar cortex and are among the smallest neurons in the brain. Cerebellar granule cells are also the most numerous neurons in the brain: in humans, estimates of their total number average around 50 billion, which means that they constitute about 3/4 of the brain's neurons.

The name granule cell has been used for a number of different types of neurons whose only common feature is that they all have very small cell bodies. Granule cells are found within the granular layer of the cerebellum, the dentate gyrus of the hippocampus, the superficial layer of the dorsal cochlear nucleus, the olfactory bulb, and the cerebral cortex.
An autapse is a chemical or electrical synapse from a neuron onto itself. It can also be described as a synapse formed by the axon of a neuron on its own dendrites, in vivo or in vitro.
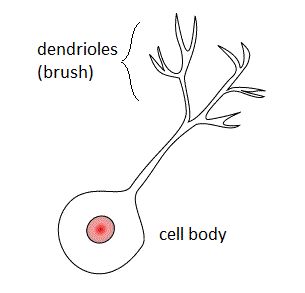
Unipolar brush cells (UBCs) are a class of excitatory glutamatergic interneuron found in the granular layer of the cerebellar cortex and also in the granule cell domain of the cochlear nucleus.

The cerebellar glomerulus is a small, intertwined mass of nerve fiber terminals in the granular layer of the cerebellar cortex. It consists of post-synaptic granule cell dendrites and pre-synaptic terminals of mossy fibers.
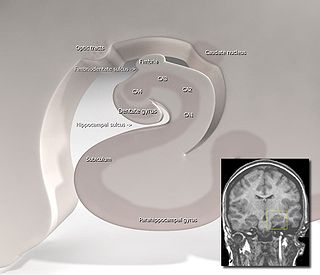
The hippocampus proper refers to the actual structure of the hippocampus which is made up of three regions or subfields. The subfields CA1, CA2, and CA3 use the initials of cornu Ammonis, an earlier name of the hippocampus.
An axo-axonic synapse is a type of synapse, formed by one neuron projecting its axon terminals onto another neuron's axon.