The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.

In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.

Tungsten is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolated as a metal in 1783. Its important ores include scheelite and wolframite, the latter lending the element its alternate name.

In crystallography, the cubiccrystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.

Tungsten(VI) oxide, also known as tungsten trioxide is a chemical compound of oxygen and the transition metal tungsten, with formula WO3. The compound is also called tungstic anhydride, reflecting its relation to tungstic acid H2WO4. It is a light yellow crystalline solid.
In chemistry, an arsenide is a compound of arsenic with a less electronegative element or elements. Many metals form binary compounds containing arsenic, and these are called arsenides. They exist with many stoichiometries, and in this respect arsenides are similar to phosphides.

Tungstic acid refers to hydrated forms of tungsten trioxide, WO3. Both a monohydrate (WO3·H2O) and hemihydrate (WO3·1/2 H2O) are known. Molecular species akin to sulfuric acid, i.e. (HO)2WO2 are not observed.

A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant.
Octahedral clusters are inorganic or organometallic cluster compounds composed of six metals in an octahedral array. Many types of compounds are known, but all are synthetic.

Sodium tungstate is the inorganic compound with the formula Na2WO4. This white, water-soluble solid is the sodium salt of tungstic acid. It is useful as a source of tungsten for chemical synthesis. It is an intermediate in the conversion of tungsten ores to the metal.

In the area of solid state chemistry. graphite intercalation compounds are materials prepared by intercalation of diverse guests into graphite. The materials have the formula (guest)Cn where n can range from 8 to 40's. The distance between the carbon layers increases significantly upon insertion of the guests. Common guests are reducing agents such as alkali metals. Strong oxidants, such as arsenic pentafluoride also intercalate into graphite. Intercalation involves electron transfer into or out of the host. The properties of these materials differ from those of the parent graphite.

Niobium dioxide, is the chemical compound with the formula NbO2. It is a bluish-black non-stoichiometric solid with a composition range of NbO1.94-NbO2.09. It can be prepared by reducing Nb2O5 with H2 at 800–1350 °C. An alternative method is reaction of Nb2O5 with Nb powder at 1100 °C.

Boron can be prepared in several crystalline and amorphous forms. Well known crystalline forms are α-rhombohedral (α-R), β-rhombohedral (β-R), and β-tetragonal (β-T). In special circumstances, boron can also be synthesized in the form of its α-tetragonal (α-T) and γ-orthorhombic (γ) allotropes. Two amorphous forms, one a finely divided powder and the other a glassy solid, are also known. Although at least 14 more allotropes have been reported, these other forms are based on tenuous evidence or have not been experimentally confirmed, or are thought to represent mixed allotropes, or boron frameworks stabilized by impurities. Whereas the β-rhombohedral phase is the most stable and the others are metastable, the transformation rate is negligible at room temperature, and thus all five phases can exist at ambient conditions. Amorphous powder boron and polycrystalline β-rhombohedral boron are the most common forms. The latter allotrope is a very hard grey material, about ten percent lighter than aluminium and with a melting point (2080 °C) several hundred degrees higher than that of steel.
In chemistry, molybdenum bronze is a generic name for certain mixed oxides of molybdenum with the generic formula A
xMo
yO
z where A may be hydrogen, an alkali metal cation (such as Li+, Na+, K+), and Tl+. These compounds form deeply coloured plate-like crystals with a metallic sheen, hence their name. These bronzes derive their metallic character from partially occupied 4d bands. The oxidation states in K0.28MoO3 are K+1, O2−, and Mo+5.72. MoO3 is an insulator, with an unfilled 4d band.
Lithium molybdenum purple bronze is a chemical compound with formula Li
0.9Mo
6O
17, that is, a mixed oxide of molybdenum and lithium. It can be obtained as flat crystals with a purple-red color and metallic sheen.
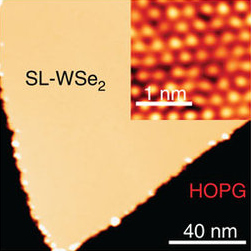
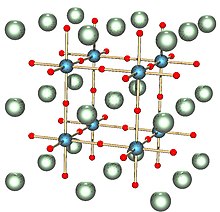
Tungsten diselenide is an inorganic compound with the formula WSe2. The compound adopts a hexagonal crystalline structure similar to molybdenum disulfide. The tungsten atoms are covalently bonded to six selenium ligands in a trigonal prismatic coordination sphere while each selenium is bonded to three tungsten atoms in a pyramidal geometry. The tungsten–selenium bond has a length of 0.2526 nm, and the distance between selenium atoms is 0.334 nm. It is a well studied example of a layered material. The layers stack together via van der Waals interactions. WSe2 is a very stable semiconductor in the group-VI transition metal dichalcogenides.

Molybdenum(IV) telluride, molybdenum ditelluride or just molybdenum telluride is a compound of molybdenum and tellurium with formula MoTe2, corresponding to a mass percentage of 27.32% molybdenum and 72.68% tellurium. It can crystallise in two dimensional sheets which can be thinned down to monolayers that are flexible and almost transparent. It is a semiconductor, and can fluoresce. It is part of a class of materials called transition metal dichalcogenides. As a semiconductor the band gap lies in the infrared region. This raises the potential use as a semiconductor in electronics or an infrared detector.
In chemistry, a hydridonitride is a chemical compound that contains hydride and nitride ions in a single phase. These inorganic compounds are distinct from inorganic amides and imides as the hydrogen does not share a bond with nitrogen, and contain a larger proportion of metals.
Samarium compounds are compounds formed by the lanthanide metal samarium (Sm). In these compounds, samarium generally exhibits the +3 oxidation state, such as SmCl3, Sm(NO3)3 and Sm(C2O4)3. Compounds with samarium in the +2 oxidation state are also known, for example SmI2.

Molybdenum difluoride dioxide is the inorganic compound with the formula MoF2O2. It is a white, diamagnetic, volatile solid.