
Gestrinone, sold under the brand names Dimetrose and Nemestran among others, is a medication which is used in the treatment of endometriosis. It has also been used to treat other conditions such as uterine fibroids and heavy menstrual bleeding and has been investigated as a method of birth control. Gestrinone is used alone and is not formulated in combination with other medications. It is taken by mouth or in through the vagina.

Norgestrienone, sold under the brand names Ogyline, Planor, and Miniplanor, is a progestin medication which has been used in birth control pills, sometimes in combination with ethinylestradiol. It was developed by Roussel Uclaf and has been registered for use only in France. Under the brand name Planor, it has been marketed in France as 2 mg norgestrienone and 50 μg ethinylestradiol tablets. It is taken by mouth.

Trestolone, also known as 7α-methyl-19-nortestosterone (MENT), is an experimental androgen/anabolic steroid (AAS) and progestogen medication which has been under development for potential use as a form of hormonal birth control for men and in androgen replacement therapy for low testosterone levels in men but has never been marketed for medical use. It is given as an implant that is placed into fat. As trestolone acetate, an androgen ester and prodrug of trestolone, the medication can also be given by injection into muscle.

Mibolerone, also known as dimethylnortestosterone (DMNT) and sold under the brand names Cheque Drops and Matenon, is a synthetic, orally active, and extremely potent anabolic–androgenic steroid (AAS) and a 17α-alkylated nandrolone (19-nortestosterone) derivative which was marketed by Upjohn for use as a veterinary drug. It was indicated specifically as an oral treatment for prevention of estrus (heat) in adult female dogs.

Promegestone, sold under the brand name Surgestone, is a progestin medication which is used in menopausal hormone therapy and in the treatment of gynecological disorders. It is taken by mouth.

Demegestone, sold under the brand name Lutionex, is a progestin medication which was previously used to treat luteal insufficiency but is now no longer marketed. It is taken by mouth.

Trimegestone, sold under the brand names Ondeva and Totelle among others, is a progestin medication which is used in menopausal hormone therapy and in the prevention of postmenopausal osteoporosis. It was also under development for use in birth control pills to prevent pregnancy, but ultimately was not marketed for this purpose. The medication is available alone or in combination with an estrogen. It is taken by mouth.

Segesterone acetate (SGA), sold under the brand name Nestorone among others, is a progestin medication which is used in birth control and in the treatment of endometriosis. It is available both alone and in combination with an estrogen as segesterone acetate/ethinylestradiol. It is not effective by mouth and must be given by other routes, most typically as a vaginal ring or implant that is placed into fat.
19-Norprogesterone, also known as 19-norpregn-4-ene-3,20-dione, is a steroidal progestin and close analogue of the sex hormone progesterone, lacking only the C19 methyl group of that molecule. It was first synthesized in 1944 in the form of a mixture that also included unnatural stereoisomers of progesterone, and this mixture was found to be at least equivalent to progesterone in terms of progestogenic activity. Subsequent investigations revealed that 17-isoprogesterone and 14-iso-17-isoprogesterone are devoid of progestogenic activity. 19-Norprogesterone was resynthesized in 1951 with an improved method, and was confirmed to be the component of the mixture synthesized in 1944 that was responsible for its progestogenic activity. In 1953, a paper was published showing that 19-norprogesterone possessed 4- to 8-fold the activity of progesterone in the Clauberg assay in rabbits, and at the time of this discovery, 19-norprogesterone was the most potent progestogen known.

Retroprogesterone, also known as 9β,10α-progesterone or as 9β,10α-pregn-4-ene-3,20-dione, is a progestin which was never marketed. It is a stereoisomer of the naturally occurring progestogen progesterone, in which the hydrogen atom at the 9th carbon is in the α-position instead of the β-position and the methyl group at the 10th carbon is in the β-position instead of the α-position. In other words, the atom positions at the two carbons have been reversed relative to progesterone, hence the name retroprogesterone. This reversal results in a "bent" configuration in which the plane of rings A and B is orientated at a 60° angle below the rings C and D. This configuration is ideal for interaction with the progesterone receptor, with retroprogesterone binding with high affinity to this receptor. However, the configuration is not as ideal for binding to other steroid hormone receptors, and as a result, retroprogesterone derivatives have increased selectivity for the progesterone receptor relative to progesterone.

Methylestradiol, sold under the brand names Ginecosid, Ginecoside, Mediol, and Renodiol, is an estrogen medication which is used in the treatment of menopausal symptoms. It is formulated in combination with normethandrone, a progestin and androgen/anabolic steroid medication. Methylestradiol is taken by mouth.

A progestogen ester is an ester of a progestogen or progestin. The prototypical progestogen is progesterone, an endogenous sex hormone. Esterification is frequently employed to improve the pharmacokinetics of steroids, including oral bioavailability, lipophilicity, and elimination half-life. In addition, with intramuscular injection, steroid esters are often absorbed more slowly into the body, allowing for less frequent administration. Many steroid esters function as prodrugs.

17α-Methylprogesterone (17α-MP), or 17α-methylpregn-4-ene-3,20-dione, is a steroidal progestin related to progesterone that was synthesized and characterized in 1949 but was never marketed. Along with ethisterone (1938) and 19-norprogesterone (1951), 17α-MP was one of the earliest derivatives of progesterone to be identified as possessing progestogenic activity. Similarly to progesterone and derivatives like 17α-hydroxyprogesterone and 19-norprogesterone, 17α-MP was found to possess poor oral bioavailability, but showed improved progestogenic activity relative to progesterone when administered via other routes. In addition to its activity as a progestogen, 17α-MP has also been found to possess some antiglucocorticoid activity.

Dimethyltrienolone is a synthetic, orally active, and extremely potent anabolic–androgenic steroid (AAS) and 17α-alkylated 19-nortestosterone (nandrolone) derivative which was never marketed for medical use. It has among the highest known affinity of any AAS for the androgen receptors, and has been said to be perhaps the most potent AAS to have ever been developed.

RU-2309, also known as 18-methylmetribolone, δ9,11-17α,18-dimethyl-19-nortestosterone, or 17α,18-dimethylestr-4,9,11-trien-17β-ol-3-one, is a 17α-alkylated androgen/anabolic steroid (AAS) of the 19-nortestosterone group which was never marketed. It is the C18 methyl or C13β ethyl derivative of metribolone. The compound is closely related to tetrahydrogestrinone (THG), which has the same chemical structure as RU-2309 except for possessing an ethyl group at the C17α position instead of a methyl group. Hence, it could also be referred to as 17α-methyl-THG. RU-2309 shows high affinity for the androgen, progesterone, and glucocorticoid receptors.
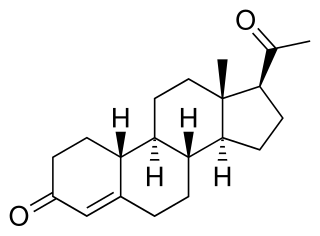
Dimethyldienolone, or 7α,17α-dimethyldienolone, also known as δ9-7α,17α-dimethyl-19-nortestosterone or as 7α,17α-dimethylestr-4,9-dien-17β-ol-3-one, is a 17α-alkylated androgen/anabolic steroid of the 19-nortestosterone group which was never marketed. It is closely related to dimethyltrienolone, as well as to mibolerone and metribolone. Dimethyldienolone shows high affinity for the androgen and progesterone receptors.
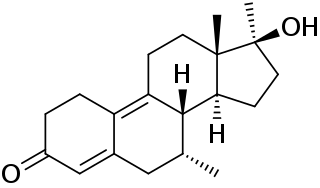
18-Methylsegesterone acetate is a progestin medication of the 19-norprogesterone group which was never marketed. It was first described in a patent in 1997 and then in a literature paper in 2003. 18-Methyl-SGA is the C18 methyl or C13β ethyl derivative of segesterone acetate, and shows 3 to 10 times the progestogenic potency of SGA in bioassays. This is analogous to the case of the 19-nortestosterone progestin norethisterone and its 18-methyl derivative levonorgestrel, the latter showing substantially increased potency relative to the former similarly. As SGA is already one of the most potent progestins to have been developed, with 100-fold the potency of progesterone and 10-fold the potency of levonorgestrel in bioassays, 18-methyl-SGA is an extremely potent progestogen, among if not the most potent known.

5α-Dihydroethisterone is an active metabolite of the formerly clinically used but now-discontinued progestin ethisterone and the experimental and never-marketed hormonal antineoplastic agent ethynylandrostanediol (HE-3235). Its formation from its parent drugs is catalyzed by 5α-reductase in tissues that express the enzyme in high amounts like the liver, skin, hair follicles, and prostate gland. 5α-DHET has significant affinity for steroid hormone receptors and may contribute importantly to the activities of its parent drugs.
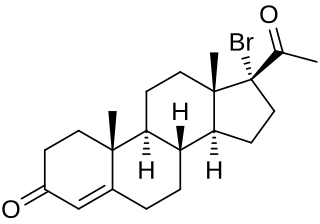
17α-Bromoprogesterone (17α-BP) is a progestin which was first described in 1957 and was never marketed. It is about twice as potent as progesterone in terms of progestogenic activity in animal bioassays. 17α-BP is a parent compound of haloprogesterone (6α-fluoro-17α-bromoprogesterone) and 6α-methyl-17α-bromoprogesterone.

16-Methylene-17α-hydroxyprogesterone acetate is a progestin of the 17α-hydroxyprogesterone group which was never marketed. Given orally, it shows about 2.5-fold the progestogenic activity of parenteral progesterone in animal bioassays. It is a parent compound of the following clinically used progestins:




















