
Heroin, also known as diacetylmorphine and diamorphine among other names, is a morphinan opioid substance synthesized from the dried latex of the Papaver somniferum plant and is mainly used as a recreational drug for its euphoric effects. Medical-grade diamorphine is used as a pure hydrochloride salt. Various white and brown powders sold illegally around the world as heroin are routinely diluted with cutting agents. Black tar heroin is a variable admixture of morphine derivatives—predominantly 6-MAM (6-monoacetylmorphine), which is the result of crude acetylation during clandestine production of street heroin. Heroin is used medically in several countries to relieve pain, such as during childbirth or a heart attack, as well as in opioid replacement therapy.

Morphine is a strong opiate that is found naturally in opium, a dark brown resin produced by drying the latex of opium poppies. It is mainly used as an analgesic. There are numerous methods used to administer morphine: oral; sublingual; via inhalation; injection into a muscle, injection under the skin, or injection into the spinal cord area; transdermal; or via rectal suppository. It acts directly on the central nervous system (CNS) to induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop with repeated administration. It can be taken for both acute pain and chronic pain and is frequently used for pain from myocardial infarction, kidney stones, and during labor. Its maximum effect is reached after about 20 minutes when administered intravenously and 60 minutes when administered by mouth, while the duration of its effect is 3–7 hours. Long-acting formulations of morphine are available as MS-Contin, Kadian, and other brand names as well as generically.

The term narcotic originally referred medically to any psychoactive compound with numbing or paralyzing properties. In the United States, it has since become associated with opiates and opioids, commonly morphine and heroin, as well as derivatives of many of the compounds found within raw opium latex. The primary three are morphine, codeine, and thebaine.

Hydromorphone, also known as dihydromorphinone, and sold under the brand name Dilaudid among others, is a morphinan opioid used to treat moderate to severe pain. Typically, long-term use is only recommended for pain due to cancer. It may be used by mouth or by injection into a vein, muscle, or under the skin. Effects generally begin within half an hour and last for up to five hours. A 2016 Cochrane review found little difference in benefit between hydromorphone and other opioids for cancer pain.
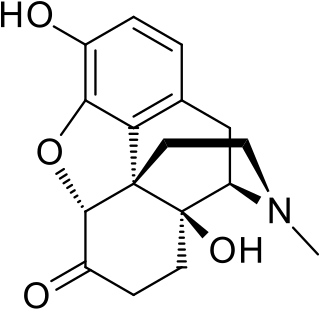
Oxymorphone is a highly potent opioid analgesic indicated for treatment of severe pain. Pain relief after injection begins after about 5–10 minutes, after oral administration it begins after about 30 minutes, and lasts about 3–4 hours for immediate-release tablets and 12 hours for extended-release tablets. The elimination half-life of oxymorphone is much faster intravenously, and as such, the drug is most commonly used orally. Like oxycodone, which metabolizes to oxymorphone, oxymorphone has a high potential to be abused.

6-Monoacetylmorphine is an opioid and also one of three active metabolites of heroin (diacetylmorphine), the others being morphine and the much less active 3-monoacetylmorphine (3-MAM).

Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others. Typical examples include compounds such as morphine, codeine, and dextromethorphan (DXM). Despite related molecular structures, the pharmacological profiles and mechanisms of action between the various types of morphinan substances can vary substantially. They tend to function either as μ-opioid receptor agonists (opioids), or NMDA receptor antagonists (dissociatives).

Thebacon, or dihydrocodeinone enol acetate, is a semisynthetic opioid that is similar to hydrocodone and is most commonly synthesised from thebaine. Thebacon was invented in Germany in 1924, four years after the first synthesis of hydrocodone. Thebacon is a derivative of acetyldihydrocodeine, where only the 6–7 double bond is saturated. Thebacon is marketed as its hydrochloride salt under the trade name Acedicon, and as its bitartrate under Diacodin and other trade names. The hydrochloride salt has a free base conversion ratio of 0.846. Other salts used in research and other settings include thebacon's phosphate, hydrobromide, citrate, hydroiodide, and sulfate.

Nicomorphine is the 3,6-dinicotinate ester of morphine. It is a strong opioid agonist analgesic two to three times as potent as morphine with a side effect profile similar to that of dihydromorphine, morphine, and diamorphine.

Nicocodeine is an opioid analgesic and cough suppressant, an ester of codeine closely related to dihydrocodeine and the codeine analogue of nicomorphine. It is not commonly used in most countries, but has activity similar to other opiates. Nicocodeine and nicomorphine were synthesized in 1904, and introduced in 1957 by Lannacher Heilmittel of Austria. Nicocodeine is metabolised in the liver by demethylation to produce nicomorphine, also known as 6-nicotinoylmorphine, and subsequently further metabolised to morphine. Side effects are similar to those of other opiates and include itching, nausea and respiratory depression. Related opioid analogues such as nicomorphine and nicodicodeine were first synthesized. The definitive synthesis, which involves treating anhydrous codeine base with nicotinic anhydride at 130 °C, was published by Pongratz and Zirm in Monatshefte für Chemie in 1957, simultaneously with the two analogues in an article about amides and esters of various organic acids.

Diacetyldihydromorphine is a potent opiate derivative developed in Germany in 1928 which is rarely used in some countries for the treatment of severe pain such as that caused by terminal cancer, as another form of diacetylmorphine. Diacetyldihydromorphine is fast-acting and longer-lasting than diamorphine, with a duration of action of around 4–7 hours.

Dipropanoylmorphine is an opiate derivative, the 3,6-dipropanoyl ester of morphine. It was developed in 1972 as an analgesic. It is rarely used in some countries for the relief of severe pain such as that caused by terminal cancer, as an alternative to diamorphine (heroin) and morphine. The drug was first synthesised circa or about 1875 in Great Britain along with many other esters of morphine, all of which were shelved at the time, some of which were later developed such as heroin (1898), acetylpropionylmorphine (1924), dibenzoylmorphine, and so on. The name of this drug is also given as 3,6-dipropanoylmorphine and its 6-mono-acetylated homologue is also a longer-acting heroin-like drug, as are 3,6-diformylmorphine and 6-formylmorphine.

Acetylmorphone is an opiate analogue that is an acetylated derivative of hydromorphone which was developed in the early 1900s as a potential cough suppressant and analgesic. It is prepared by the acetylation of hydromorphone using either acetyl chloride or acetic anhydride. It was banned internationally in 1930 by the Health Committee of the League of Nations, in order to prevent its sale as an analogue of heroin.
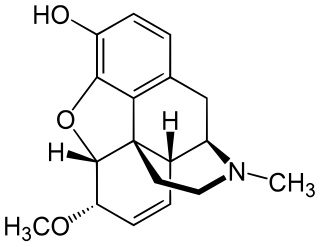
Heterocodeine (6-methoxymorphine) is an opiate derivative, the 6-methyl ether of morphine, and a structural isomer of codeine; it is called "hetero-" because it is the reverse isomer of codeine. Heterocodeine was first synthesised in 1932 and first patented in 1935. It can be made from morphine by selective methylation. Codeine is the natural mono-methyl ether, but must be metabolized for activity. In contrast the semi-synthetic mono-methyl ether, heterocodeine is a direct agonist. The 6,7,8,14 tetradehydro 3,6 methyl di-ether of morphine is thebaine.

Chloromorphide (α-chloromorphide) is an opiate analog that is a derivative of morphine, where the 6-hydroxy group has been replaced by chlorine. Developed in 1933 in Germany, it has approximately ten times the potency of morphine. It has similar effects to morphine, such as sedation, analgesia, and respiratory depression.

An opiate is an alkaloid substance derived from opium It has a different meaning from the similar term opioid, used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain. Opiates are alkaloid compounds naturally found in the opium poppy plant Papaver somniferum. The psychoactive compounds found in the opium plant include morphine, codeine, and thebaine. Opiates have long been used for a variety of medical conditions, with evidence of opiate trade and use for pain relief as early as the eighth century AD. Most opiates are considered drugs with moderate to high abuse potential and are listed on various "Substance-Control Schedules" under the Uniform Controlled Substances Act of the United States of America.

Dibenzoylmorphine is an opiate analogue that is a derivative of morphine. It was developed in the early 1900s after first having been synthesised in 1875 in the UK by the CR Alders Wright organisation at Bayer, along with various other esters of morphine. It was never used medically, instead being widely sold as one of the first "designer drugs" for around five years following the introduction of the first international restrictions on the sale of heroin in 1925. It is described as being virtually identical to heroin and morphine in its effects, and consequently was itself banned internationally in 1930 by the Health Committee of the League of Nations, in order to prevent its sale as an unscheduled alternative to diacetylmorphine. However, it still continues to occasionally be encountered as a result of home manufacture from morphine by drug users.

Acetylpropionylmorphine is an opiate analog that is an ester of morphine. It was developed in the early 1900s after first being synthesized in Great Britain in 1875 but shelved along with heroin and various other esters of morphine. Acetylpropionylmorphone was never used medically, instead being widely sold as one of the first "designer drugs" for around five years following the introduction of the first international restrictions on the sale of heroin in 1925. It is described as being virtually identical to heroin and morphine in its effects, and consequently was itself banned internationally in 1930 by the Health Committee of the League of Nations, in order to prevent its sale as an unscheduled alternative to heroin.

1-Iodomorphine is a semi-synthetic narcotic analgesic formed by halogenation of the 1 position on the morphine carbon skeleton. Halogenated morphine derivatives were first synthesised in Germany, Austria/Austria-Hungary, the United Kingdom and the United States in the period 1890 to 1930. Use of this drug increased after 1945 for the below-mentioned research. It is a research chemical which is often prepared in the laboratory when it is needed.

Dibutyrylmorphine is the 3,6-dibutyryl ester of morphine, first synthesized by the CR Alders Wright organization in the United Kingdom in 1875.





















