
Morphine, formerly also called morphia, is an opiate that is found naturally in opium, a dark brown resin produced by drying the latex of opium poppies. It is mainly used as an analgesic. There are numerous methods used to administer morphine: orally; administered under the tongue; via inhalation; injection into a vein, injection into a muscle, injection under the skin, or injection into the spinal cord area; transdermal; or via administered into the rectal canal suppository. It acts directly on the central nervous system (CNS) to induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop with repeated administration. It can be taken for both acute pain and chronic pain and is frequently used for pain from myocardial infarction, kidney stones, and during labor. Its maximum effect is reached after about 20 minutes when administered intravenously and 60 minutes when administered by mouth, while the duration of its effect is 3–7 hours. Long-acting formulations of morphine are sold under the brand names MS Contin and Kadian, among others. Generic long-acting formulations are also available.

Epibatidine is a chlorinated alkaloid that is secreted by the Ecuadoran frog Epipedobates anthonyi and poison dart frogs from the Ameerega genus. It was discovered by John W. Daly in 1974, but its structure was not fully elucidated until 1992. Whether epibatidine occurs naturally remains controversial due to challenges in conclusively identifying the compound from the limited samples collected by Daly. By the time that high-resolution spectrometry was used in 1991, there remained less than one milligram of extract from Daly's samples, raising concerns about possible contamination. Samples from other batches of the same species of frog failed to yield epibatidine.

Ohmefentanyl is an extremely potent opioid analgesic drug which selectively binds to the μ-opioid receptor.

Oripavine is an opioid and the major metabolite of thebaine. It is the precursor to the semi-synthetic compounds etorphine and buprenorphine. Although this chemical compound has analgesic potency comparable to morphine, it is not used clinically due to severe adverse effects and a low therapeutic index. Being a precursor to a series of extremely strong opioids, oripavine is a controlled substance in some jurisdictions.
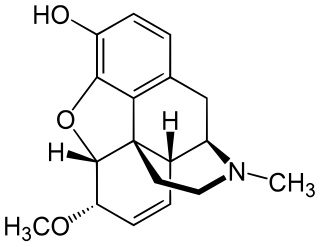
Heterocodeine (6-methoxymorphine) is an opiate derivative, the 6-methyl ether of morphine, and a structural isomer of codeine; it is called "hetero-" because it is the reverse isomer of codeine. Heterocodeine was first synthesised in 1932 and first patented in 1935. It can be made from morphine by selective methylation. Codeine is the natural mono-methyl ether, but must be metabolized for activity. In contrast the semi-synthetic mono-methyl ether, heterocodeine is a direct agonist. The 6,7,8,14 tetradehydro 3,6 methyl di-ether of morphine is thebaine.
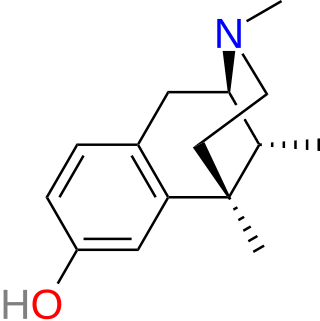
Metazocine is an opioid analgesic related to pentazocine. While metazocine has significant analgesic effects, mediated through a mixed agonist–antagonist action at the mu opioid receptor, its clinical use is limited by dysphoric and hallucinogenic effects which are most likely caused by activity at kappa opioid receptors and/or sigma receptors.

Enadoline is a drug which acts as a highly selective κ-opioid agonist.

Desomorphine is a semi-synthetic opioid commercialized by Roche, with powerful, fast-acting effects, such as sedation and analgesia. It was first discovered and patented in Germany by a German team working for Knoll in 1920 but was not generally recognized. It was later synthesized in 1932 by American chemist Lyndon Frederick Small. Small also successfully patented it in 1934 in the United States. Desomorphine was used in Germany, Austria, and Switzerland under the brand name Permonid and was described as having a fast onset and a short duration of action, with relatively little nausea compared to equivalent doses of morphine. Dose for dose it is roughly ten times more potent than morphine, with 1 mg desomorphine being equivalent 10 mg morphine, via the intravenous (IV) or intramuscular (IM) routes.

14-Phenylpropoxymetopon (PPOM) is an opioid analogue that is a derivative of metopon which has been substituted with a γ-phenylpropoxy group at the 14-position. PPOM is a highly potent analgesic drug several thousand times stronger than morphine, with an even higher in vivo potency than etorphine. The 14-phenylpropoxy substitution appears to confer potent μ-opioid agonist activity, even when combined with substitutions such as N-cyclopropyl or N-allyl, which normally result in μ-opioid antagonist compounds.

N-Phenethyl-14-ethoxymetopon is a drug that is a derivative of metopon. It is a potent analgesic, around 60 times stronger than morphine and produces significantly less constipation.

Mirfentanil is a fentanyl derivative with strong selectivity for the μ opioid receptor. At lower doses, it antagonizes the analgesic effects of alfentanil and substitutes for naloxone in morphine-treated monkeys; however, it also reverses naloxone-precipitated withdrawal in pigeons trained to discriminate morphine from naloxone.

Azaprocin is a drug which is an opioid analgesic with approximately ten times the potency of morphine, and a fast onset and short duration of action. It was discovered in 1963, but has never been marketed.

Xorphanol (INN), also known as xorphanol mesylate (USAN), is an opioid analgesic of the morphinan family that was never marketed.
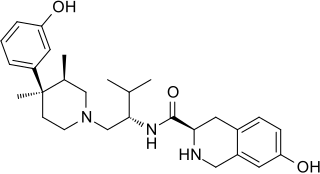
JDTic is a selective, long-acting ("inactivating") antagonist of the κ-opioid receptor (KOR). JDTic is a 4-phenylpiperidine derivative, distantly related structurally to analgesics such as pethidine and ketobemidone, and more closely to the MOR antagonist alvimopan. In addition, it is structurally distinct from other KOR antagonists such as norbinaltorphimine. JDTic has been used to create crystal structures of KOR [ PDB: 4DJH, 6VI4].

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.

MT-45 (IC-6) is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co. It is chemically a 1-substituted-4-(1,2-diphenylethyl) piperazine derivative, which is structurally unrelated to most other opioid drugs. Racemic MT-45 has around 80% the potency of morphine, with almost all opioid activity residing in the (S) enantiomer. It has been used as a lead compound from which a large family of potent opioid drugs have been developed, including full agonists, partial agonists, and antagonists at the three main opioid receptor subtypes. Fluorinated derivatives of MT-45 such as 2F-MT-45 are significantly more potent as μ-opioid receptor agonists, and one of its main metabolites 1,2-diphenylethylpiperazine also blocks NMDA receptors.

Conolidine is an indole alkaloid. Preliminary reports suggest that it could provide analgesic effects with few of the detrimental side-effects associated with opioids such as morphine, though at present it has only been evaluated in mouse models.
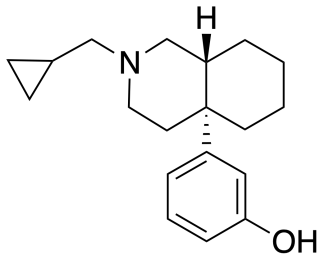
Ciprefadol is an opioid analgesic that is an isoquinoline derivative most closely related to cyclazocine and picenadol, with a number of other related compounds known. Ciprefadol is a mixed agonist–antagonist at μ-opioid receptors and can partly block the effects of morphine at low doses, though at higher doses it acts more like a full agonist. It is also a potent κ-opioid agonist, unlike the corresponding N-methyl and N-phenethyl derivatives which are reasonably μ-selective agonists.

Mitragynine pseudoindoxyl is a rearrangement product of 7-hydroxymitragynine, an active metabolite of mitragynine.

HS665 is a drug which acts as a potent and selective κ-opioid receptor agonist, and has analgesic effects in animal studies. HS665 is not an agonist for the mu receptor, leading to less potential for abuse.




















