
Etonitazene, also known as EA-4941 or CS-4640, is a benzimidazole opioid, first reported in 1957, that has been shown to have approximately 1,000 to 1,500 times the potency of morphine in animals.

14-Phenylpropoxymetopon (PPOM) is an opioid analogue that is a derivative of metopon which has been substituted with a γ-phenylpropoxy group at the 14-position. PPOM is a highly potent analgesic drug several thousand times stronger than morphine, with an even higher in vivo potency than etorphine. The 14-phenylpropoxy substitution appears to confer potent μ-opioid agonist activity, even when combined with substitutions such as N-cyclopropyl or N-allyl, which normally result in μ-opioid antagonist compounds.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.

Biphalin is a dimeric enkephalin endogenous peptide (Tyr-D-Ala-Gly-Phe-NH)2 composed of two tetrapeptides derived from enkephalins, connected 'tail-to-tail' by a hydrazide bridge. The presence of two distinct pharmacophores confers on biphalin a high affinity for both μ and δ opioid receptors (with an EC50 of about 1–5 nM for both μ and δ receptors), therefore it has analgesic activity. Biphalin presents a considerable antinociceptive profile. In fact, when administered intracerebroventricularly in mice, biphalin displays a potency almost 7-fold greater than that of the ultra-potent alkaloid agonist, etorphine and 7000-fold greater than morphine; biphalin and morphine were found to be equipotent after intraperitoneal administration. The extraordinary in vivo potency shown by this compound is coupled with low side-effects, in particular, to produce no dependency in chronic use. For these reasons, several efforts have been carried out in order to obtain more information about structure-activity relationship (SAR). Results clearly indicate that, at least for μ receptor binding, the presence of two pharmacophores is not necessary; Tyr1 is indispensable for analgesic activity, while replacing Phe at the position 4 and 4' with non-aromatic, but lipophilic amino acids does not greatly change the binding properties and in general 4,4' positions are found to be important to design biphalin analogues with increased potency and modified μ/δ selectivity. The hydrazide linker is not fundamental for activity or binding, and it can be conveniently substituted by different conformationally constrained cycloaliphatic diamine linkers.

Metonitazene is an analgesic compound related to etonitazene, which was first reported in 1957, and has been shown to have approximately 1000 times the potency of morphine by central routes of administration, but if used orally it has been shown to have approximately 10 times the potency of morphine.

Isotonitazene is a benzimidazole-derived opioid analgesic drug related to etonitazene, which has been sold as a designer drug. It has only around half the potency of etonitazene in animal studies, but it is likely even less potent in humans as was seen with etonitazene. Isotonitazene was fully characterized in November 2019 in a paper where the authors performed a full analytical structure elucidation in addition to determination of the potency at the μ-opioid receptor using a biological functional assay in vitro. While isotonitazene was not compared directly to morphine in this assay, it was found to be around 2.5 times more potent than hydromorphone and slightly more potent than fentanyl.

Brorphine is a piperidine-based opioid analgesic compound. Brorphine was originally discovered in a 2018 paper investigating functionally biased opioid compounds, with the intention of finding safer analgesics that produce less respiratory depression than typical opioids. Brorphine was originally reported to be highly biased, with an EC50 of 4.8nM for GTPγS binding and 182nM for β-arrestin recruitment, however a more recent study found no significant bias for any of the compounds tested, including brorphine. Its safety profile in any animal model has never been established. Despite the lack of safety information on the compound, brorphine has been sold as a designer drug since mid-2019, initially being identified in the US Midwest, though it has since been found in 2020 in Belgium. It is related in chemical structure to compounds such as benzylfentanyl and bezitramide, though it is sufficiently structurally distinct to fall outside the formal definition of a "fentanyl analogue" in jurisdictions such as the US and New Zealand which have Markush structure controls over this family of drugs.

Etodesnitazene is a benzimidazole-derived opioid analgesic drug, which was originally developed in the late 1950s alongside etonitazene and a range of related derivatives. It is many times less potent than etonitazene itself, but still 70 times more potent than morphine in animal studies. Corresponding analogues where the N,N-diethyl group is replaced by piperidine or pyrrolidine rings also retain significant activity. Etodesnitazene has been sold as a designer drug, first being identified in both Poland and Finland in March 2020.
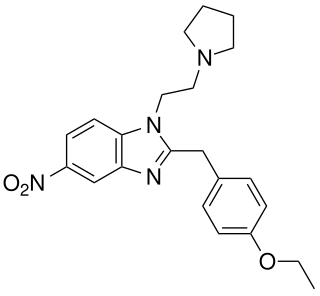
Etonitazepyne is a benzimidazole derivative with potent opioid effects which has been sold over the internet as a designer drug and linked to numerous cases of overdose.
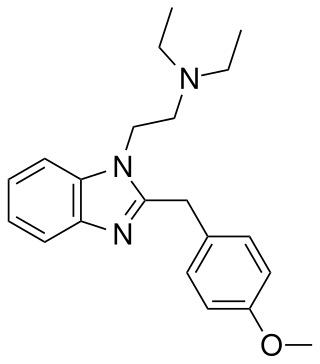
Metodesnitazene is a benzimidazole derivative with opioid effects, though unlike related compounds such as metonitazene and etodesnitazene which are quite potent, metodesnitazene is only around the same potency as morphine in animal studies. It is illegal in both the US and UK.

Butonitazene is a benzimidazole derivative with opioid effects, which has been sold over the internet as a designer drug. It has relatively low potency compared to many related compounds, and has generally been encountered as a component of mixtures with other substances rather than in its pure form. However, it is still several times the potency of morphine and has been implicated in several cases of drug overdose. Butonitazene is a Schedule I drug in the US, along with several related compounds.

N-Desethylisotonitazene (norisotonitazene) is a benzimidazole opioid with potent analgesic effects which has been sold as a designer drug. It was first identified in 2023 as an active metabolite of the closely related compound isotonitazene, and was found to have similar potency. It is one of the strongest benzimidazole opioids discovered, with an analgesic strength 20 times stronger than fentanyl.

Etomethazene (5-methyldesnitroetonitazene, 5-methyl etodesnitazene, Eto) is a benzimidazole derivative with opioid effects which has been sold as a designer drug over the internet since 2022, first being definitively identified in Sweden in January 2023. It is an analogue of etonitazene where the nitro (NO2) group has been replaced by a methyl (CH3) group. While formal studies into its pharmacology have yet to be carried out, it showed far less potency than etonitazene itself. Etomethazene has an analgesic potency around 20 times that of morphine with a relatively short duration of about 120 min.

Protonitazepyne is a benzimidazole derivative with opioid effects, which has been sold as a designer drug over the internet, first being mentioned in mid 2022 and definitively identified in drug seizures in Canada in early 2023 and Ireland in late 2023. It is an analogue of etonitazene where the ethoxy group has been extended to propoxy, and the N,N-diethyl substitution has been cyclised into a pyrrolidine ring. While formal studies into its pharmacology have yet to be carried out, it is believed to be slightly less potent than the ethoxy analogue etonitazepyne but still a potent opioid.

Etonitazene 5-cyano analogue (Etocyanazene, 5-cyanodesnitroetonitazene) is a benzimidazole derivative with opioid effects, first developed in the 1950s as part of the research that led to better-known compounds such as etonitazene. It is an analogue of etonitazene where the 5-nitro (NO2) group has been replaced by a nitrile (C≡N) group. It is described as having "reduced but still significant" potency compared to etonitazene itself. It was made illegal in Germany in July 2021.
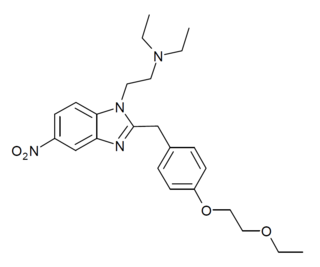
Etoetonitazene is a benzimidazole derivative with opioid effects, first developed in the 1950s as part of the research that led to better-known compounds such as etonitazene. It is an analogue of etonitazene where the ethoxy sidechain has been extended to ethoxyethoxy. It is less potent than other benzimidazole class opioids, but is still a potent mu opioid receptor agonist with around 50x the potency of morphine, and has been sold as a designer drug since around 2022.

Flunitazene (Fluonitazene) is a benzimidazole derivative with opioid effects, first developed in the 1950s as part of the research that led to better-known compounds such as etonitazene. It is one of the least potent derivatives from this class to have appeared as a designer drug, with only around the same potency as morphine, but nevertheless has been sold since around 2020, and has been linked to numerous drug overdose cases.

















