
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.

Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.

Manganese dioxide is the inorganic compound with the formula MnO
2. This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for MnO
2 is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery. MnO
2 is also used as a pigment and as a precursor to other manganese compounds, such as KMnO
4. It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. MnO
2 has an α-polymorph that can incorporate a variety of atoms in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in α-MnO
2 as a possible cathode for lithium-ion batteries.

Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. It contains manganese (Mn), technetium (Tc), rhenium (Re) and bohrium (Bh). This group lies in the d-block of the periodic table, and are hence transition metals. This group is sometimes called the manganese group or manganese family after its lightest member; however, the group itself has not acquired a trivial name because it belongs to the broader grouping of the transition metals.

Manganese(II) chloride is the dichloride salt of manganese, MnCl2. This inorganic chemical exists in the anhydrous form, as well as the dihydrate (MnCl2·2H2O) and tetrahydrate (MnCl2·4H2O), with the tetrahydrate being the most common form. Like many Mn(II) species, these salts are pink, with the paleness of the color being characteristic of transition metal complexes with high spin d5 configurations.

A permanganate is a chemical compound with the manganate(VII) ion, MnO−
4, the conjugate base of permanganic acid. Because the manganese atom has a +7 oxidation state, the permanganate(VII) ion is a strong oxidising agent. The ion is a transition metal ion with a tetrahedral structure. Permanganate solutions are purple in colour and are stable in neutral or slightly alkaline media. The exact chemical reaction depends on the carbon-containing reactants present and the oxidant used. For example, trichloroethane (C2H3Cl3) is oxidised by permanganate ions to form carbon dioxide (CO2), manganese dioxide (MnO2), hydrogen ions (H+), and chloride ions (Cl−).

In inorganic nomenclature, a manganate is any negatively charged molecular entity with manganese as the central atom. However, the name is usually used to refer to the tetraoxidomanganate(2−) anion, MnO2−
4, also known as manganate(VI) because it contains manganese in the +6 oxidation state. Manganates are the only known manganese(VI) compounds.

Iron (II) hydroxide or ferrous hydroxide is an inorganic compound with the formula Fe(OH)2. It is produced when iron (II) salts, from a compound such as iron(II) sulfate, are treated with hydroxide ions. Iron(II) hydroxide is a white solid, but even traces of oxygen impart a greenish tinge. The air-oxidised solid is sometimes known as "green rust".

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.
The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula UO2+
2. It has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands may be bound to the uranyl ion in an equatorial plane around the uranium atom. The uranyl ion forms many complexes, particularly with ligands that have oxygen donor atoms. Complexes of the uranyl ion are important in the extraction of uranium from its ores and in nuclear fuel reprocessing.
In chemistry, hypomanganate, also called manganate(V) or tetraoxidomanganate(3−), is a trivalent anion (negative ion) composed of manganese and oxygen, with formula MnO3−
4.
Potassium hypomanganate is the inorganic compound with the formula K3MnO4. Also known as potassium manganate(V), this bright blue solid is a rare example of a salt with the hypomanganate or manganate(V) anion, where the manganese atom is in the +5 oxidation state. It is an intermediate in the production of potassium permanganate and the industrially most important Mn(V) compound.

Dimanganese decacarbonyl, which has the chemical formula Mn2(CO)10, is a binary bimetallic carbonyl complex centered around the first row transition metal manganese. The first reported synthesis of Mn2(CO)10 was in 1954 at Linde Air Products Company and was performed by Brimm, Lynch, and Sesny. Their hypothesis about, and synthesis of, dimanganese decacarbonyl was fundamentally guided by the previously known dirhenium decacarbonyl (Re2(CO)10), the heavy atom analogue of Mn2(CO)10. Since its first synthesis, Mn2(CO)10 has been use sparingly as a reagent in the synthesis of other chemical species, but has found the most use as a simple system on which to study fundamental chemical and physical phenomena, most notably, the metal-metal bond. Dimanganese decacarbonyl is also used as a classic example to reinforce fundamental topics in organometallic chemistry like d-electron count, the 18-electron rule, oxidation state, valency, and the isolobal analogy.

Ferric acetate is the iron compound with the formula Fe3O(O2CCH3)6(H2O)3]O2CCH3. This red brown solid is the acetate salt of the coordination complex [Fe3O(OAc)6(H2O)3]+ (OAc− is CH3CO2−). Commonly, the salt is known as "basic iron acetate". The formation of the red-brown complex was once used as a test for ferric ions.

Layered double hydroxides (LDH) are a class of ionic solids characterized by a layered structure with the generic layer sequence [AcB Z AcB]n, where c represents layers of metal cations, A and B are layers of hydroxide anions, and Z are layers of other anions and neutral molecules. Lateral offsets between the layers may result in longer repeating periods.

Cobalt(II) hydroxide or cobaltous hydroxide is the inorganic compound with the formula Co(OH)
2, consisting of divalent cobalt cations Co2+
and hydroxide anions OH−
. The pure compound, often called the "beta form" is a pink solid insoluble in water.

Sodium bismuthate is an inorganic compound, and a strong oxidiser with chemical formula NaBiO3. It is somewhat hygroscopic, but not soluble in cold water, which can be convenient since the reagent can be easily removed after the reaction. It is one of the few water insoluble sodium salts. Commercial samples may be a mixture of bismuth(V) oxide, sodium carbonate and sodium peroxide.
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion (CH
3COCHCOCH−
3) and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCOR′−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. C
5H
7O−
2 in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have the general stoichiometry [M(H2O)n]z+. Their behavior underpins many aspects of environmental, biological, and industrial chemistry. This article focuses on complexes where water is the only ligand, but of course many complexes are known to consist of a mix of aquo and other ligands.
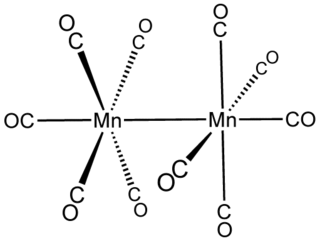
In inorganic chemistry, metal–metal bonds describe attractive interactions between metal centers. The simplest examples are found in bimetallic complexes. Metal–metal bonds can be "supported", i.e. be accompanied by one or more bridging ligands, or "unsupported". They can also vary according to bond order. The topic of metal–metal bonding is usually discussed within the framework of coordination chemistry, but the topic is related to extended metallic bonding, which describes interactions between metals in extended solids such as bulk metals and metal subhalides.














