
Muscarine, L-(+)-muscarine, or muscarin is a natural product found in certain mushrooms, particularly in Inocybe and Clitocybe species, such as the deadly C. dealbata. Mushrooms in the genera Entoloma and Mycena have also been found to contain levels of muscarine which can be dangerous if ingested. Muscarine has been found in harmless trace amounts in Boletus, Hygrocybe, Lactarius and Russula. Trace concentrations of muscarine are also found in Amanita muscaria, though the pharmacologically more relevant compound from this mushroom is the Z-drug-like alkaloid muscimol. A. muscaria fruitbodies contain a variable dose of muscarine, usually around 0.0003% fresh weight. This is very low and toxicity symptoms occur very rarely. Inocybe and Clitocybe contain muscarine concentrations up to 1.6%.

Ergoline is a chemical compound whose structural skeleton is contained in a variety of alkaloids, referred to as ergoline derivatives or ergoline alkaloids. Ergoline alkaloids, one being ergine, were initially characterized in ergot. Some of these are implicated in the condition ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives.
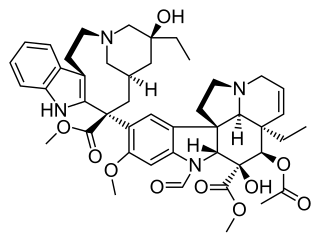
Vinca alkaloids are a set of anti-mitotic and anti-microtubule alkaloid agents originally derived from the periwinkle plant Catharanthus roseus and other vinca plants. They block beta-tubulin polymerization in a dividing cell.

Aporphine is an alkaloid with the chemical formula C17H17N. The IUPAC name of aporphine is 6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline. It is the core chemical substructure of the aporphine alkaloids, a subclass of quinoline alkaloids. It can exist in either of two enantiomeric forms, (R)-aporphine and (S)-aporphine.

Stephania is a genus of flowering plants in the family Menispermaceae, native to eastern and southern Asia and Australia. They are herbaceous perennial vines growing to around four metres tall, with a large, woody caudex. The leaves are arranged spirally on the stem, and are peltate, with the leaf petiole attached near the centre of the leaf. The name Stephania comes from the Greek, "a crown". This refers to the anthers being arranged in a crown like manner.
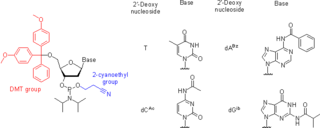
Nucleoside phosphoramidites are derivatives of natural or synthetic nucleosides. They are used to synthesize oligonucleotides, relatively short fragments of nucleic acid and their analogs. Nucleoside phosphoramidites were first introduced in 1981 by Beaucage and Caruthers. To avoid undesired side reactions, reactive hydroxy and exocyclic amino groups present in natural or synthetic nucleosides are appropriately protected. As long as a nucleoside analog contains at least one hydroxy group, the use of the appropriate protecting strategy allows one to convert that to the respective phosphoramidite and to incorporate the latter into synthetic nucleic acids. To be incorporated in the middle of an oligonucleotide chain using phosphoramidite strategy, the nucleoside analog must possess two hydroxy groups or, less often, a hydroxy group and another nucleophilic group (amino or mercapto). Examples include, but are not limited to, alternative nucleotides, LNA, morpholino, nucleosides modified at the 2'-position (OMe, protected NH2, F), nucleosides containing non-canonical bases (hypoxanthine and xanthine contained in natural nucleosides inosine and xanthosine, respectively, tricyclic bases such as G-clamp, etc.) or bases derivatized with a fluorescent group or a linker arm.
Solenopsin is a lipophilic alkaloid with the molecular formula C17H35N found in the venom of fire ants (Solenopsis). It is considered the primary toxin in the venom and may be the component responsible for the cardiorespiratory failure in people who experience excessive fire ant stings.
The Hofmann–Löffler reaction (also referred to as Hofmann–Löffler–Freytag reaction, Löffler–Freytag reaction, Löffler–Hofmann reaction, as well as Löffler's method) is an organic reaction in which a cyclic amine 2 (pyrrolidine or, in some cases, piperidine) is generated by thermal or photochemical decomposition of N-halogenated amine 1 in the presence of a strong acid (concentrated sulfuric acid or concentrated CF3CO2H). The Hofmann–Löffler–Freytag reaction proceeds via an intramolecular hydrogen atom transfer to a nitrogen-centered radical and is an example of a remote intramolecular free radical C–H functionalization.

Aquayamycin is an anthraquinone derivative. It is an inhibitor of the enzyme tyrosine hydroxylase.

Tetrandrine, a bis-benzylisoquinoline alkaloid, is a calcium channel blocker. It is isolated from the plant Stephania tetrandra, and other Chinese and Japanese herbs.
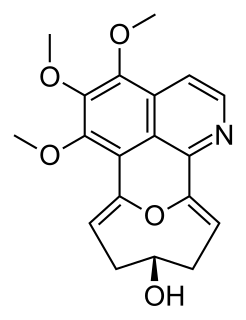
Eletefine is an isoquinoline alkaloid first isolated in 1998 from Cissampelos glaberrima. It is one of few known compounds containing the so-called stephaoxocane skeleton, alongside stephaoxocanidine, excentricine, and the stephalonganines.
The imine Diels–Alder reaction involves the transformation of all-carbon dienes and imine dienophiles into tetrahydropyridines.
A bridged nucleic acid (BNA) is a modified RNA nucleotide. They are sometimes also referred to as constrained or inaccessible RNA molecules. BNA monomers can contain a five-membered, six-membered or even a seven-membered bridged structure with a "fixed" C3'-endo sugar puckering. The bridge is synthetically incorporated at the 2', 4'-position of the ribose to afford a 2', 4'-BNA monomer. The monomers can be incorporated into oligonucleotide polymeric structures using standard phosphoamidite chemistry. BNAs are structurally rigid oligo-nucleotides with increased binding affinities and stability.

Cepharanthine is an antiinflammatory and antineoplastic compound isolated from Stephania. Due to these modalities, it has been shown effective against HTLV in lab research. Additionally, it has successfully been used to treat a diverse range of medical conditions, including radiation-induced leukopenia, idiopathic thrombocytopenic purpura, alopecia areata, alopecia pityrodes, venomous snakebites, xerostomia, sarcoidosis, refractory anemia and various cancer-related conditions. No safety issues have been observed with CEP, and side effects are very rarely reported.
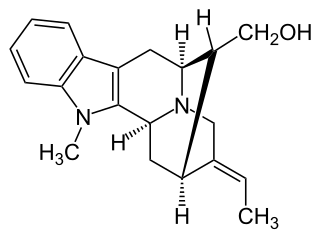
Affinisine is a monoterpenoid indole alkaloid which can be isolated from plants of the genus Tabernaemontana. Structurally, it can be considered a member of the sarpagine alkaloid family and may be synthesized from tryptophan via a Pictet-Spengler reaction.
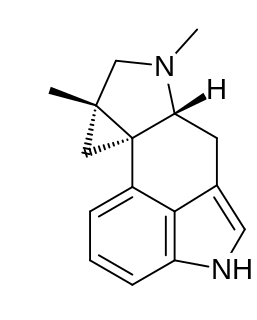
Cycloclavine is an ergot alkaloid. It was first isolated in 1969 from seeds of Ipomoea hildebrandtii vatke. The first total synthesis of (±)-cycloclavine was published in 2008 by Szántay. Further reports came from Wipf and Petronijevic, Cao and Brewer. In 2016, Wipf and McCabe completed an 8-step asymmetric synthesis of (–)-cycloclavine, and in 2018, they expanded this approach toward (+)-cycloclavine and a biological characterization of the binding profile of both enantiomers on 16 brain receptors. Natural (+)- and unnatural (–)-cycloclavine demonstrated significant stereospecificity and unique binding profiles in comparison to LSD, psilocin, and DMT. Differential 5-HT receptor affinities, as well as novel sigma-1 receptor properties, suggest potential future therapeutic opportunities of clavine alkaloid scaffolds.
Dewan Singh Bhakuni is an Indian natural product chemist, stereochemist and a former director general-grade scientist of the Central Drug Research Institute. He is known for his researches on the biogenesis of alkaloids and is an elected fellow of the Indian Academy of Sciences, the National Academy of Sciences, India and the Indian National Science Academy. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1975, for his contributions to chemical sciences.

Vobasine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.

Pseudoceratina is a genus of sponge within the family Pseudoceratinidae. They are characterized by possession of a dendritic fiber skeleton lacking laminar bark but containing pith. They have been found in a variety of habitats including the Great Barrier reef, the Red Sea, and Jamaica. Sponges of this genus have a microbiome known to produce a variety of chemicals that are used in pharmaceutical and anti-fouling activities. Notably, a species in this genus produces a chemical that is effective in inhibiting the migration of metastatic breast cancer cells.














