
The cingulate cortex is a part of the brain situated in the medial aspect of the cerebral cortex. The cingulate cortex includes the entire cingulate gyrus, which lies immediately above the corpus callosum, and the continuation of this in the cingulate sulcus. The cingulate cortex is usually considered part of the limbic lobe.

In the human brain, the anterior cingulate cortex (ACC) is the frontal part of the cingulate cortex that resembles a "collar" surrounding the frontal part of the corpus callosum. It consists of Brodmann areas 24, 32, and 33.

In neuroanatomy, the precuneus is the portion of the superior parietal lobule on the medial surface of each brain hemisphere. It is located in front of the cuneus. The precuneus is bounded in front by the marginal branch of the cingulate sulcus, at the rear by the parieto-occipital sulcus, and underneath by the subparietal sulcus. It is involved with episodic memory, visuospatial processing, reflections upon self, and aspects of consciousness.

Brodmann area 9, or BA9, refers to a cytoarchitecturally defined portion of the frontal cortex in the brain of humans and other primates. It contributes to the dorsolateral and medial prefrontal cortex.
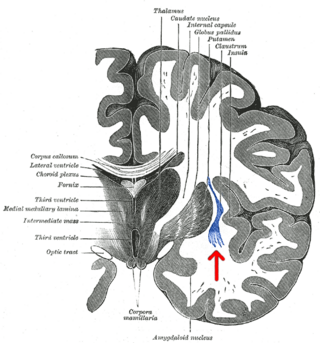
The claustrum is a thin sheet of neurons and supporting glial cells, that connects to the cerebral cortex and subcortical regions including the amygdala, hippocampus and thalamus of the brain. It is located between the insular cortex laterally and the putamen medially, encased by the extreme and external capsules respectively. Blood to the claustrum is supplied by the middle cerebral artery. It is considered to be the most densely connected structure in the brain, and thus hypothesized to allow for the integration of various cortical inputs such as vision, sound and touch, into one experience. Other hypotheses suggest that the claustrum plays a role in salience processing, to direct attention towards the most behaviorally relevant stimuli amongst the background noise. The claustrum is difficult to study given the limited number of individuals with claustral lesions and the poor resolution of neuroimaging.

Brodmann area 22 is a Brodmann's area that is cytoarchitecturally located in the posterior superior temporal gyrus of the brain. In the left cerebral hemisphere, it is one portion of Wernicke's area. The left hemisphere BA22 helps with generation and understanding of individual words. On the right side of the brain, BA22 helps to discriminate pitch and sound intensity, both of which are necessary to perceive melody and prosody. Wernicke's area is active in processing language and consists of the left Brodmann area 22 and Brodmann area 40, the supramarginal gyrus.

Brodmann area 25 (BA25) is the subgenual area, area subgenualis or subgenual cingulate area in the cerebral cortex of the brain and delineated based on its cytoarchitectonic characteristics.
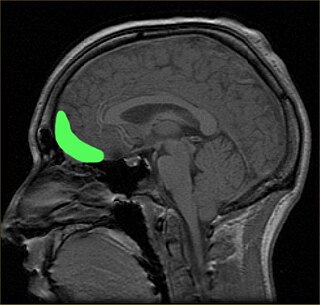
The orbitofrontal cortex (OFC) is a prefrontal cortex region in the frontal lobes of the brain which is involved in the cognitive process of decision-making. In non-human primates it consists of the association cortex areas Brodmann area 11, 12 and 13; in humans it consists of Brodmann area 10, 11 and 47.

The superior longitudinal fasciculus (SLF) is an association tract in the brain that is composed of three separate components. It is present in both hemispheres and can be found lateral to the centrum semiovale and connects the frontal, occipital, parietal, and temporal lobes. This bundle of tracts (fasciculus) passes from the frontal lobe through the operculum to the posterior end of the lateral sulcus where they either radiate to and synapse on neurons in the occipital lobe, or turn downward and forward around the putamen and then radiate to and synapse on neurons in anterior portions of the temporal lobe.
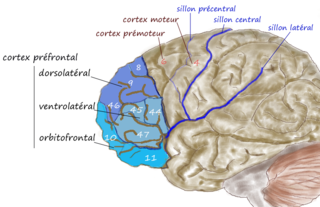
The dorsolateral prefrontal cortex is an area in the prefrontal cortex of the primate brain. It is one of the most recently derived parts of the human brain. It undergoes a prolonged period of maturation which lasts into adulthood. The DLPFC is not an anatomical structure, but rather a functional one. It lies in the middle frontal gyrus of humans. In macaque monkeys, it is around the principal sulcus. Other sources consider that DLPFC is attributed anatomically to BA 9 and 46 and BA 8, 9 and 10.
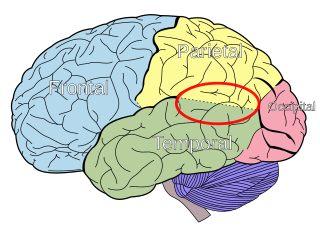
The temporoparietal junction (TPJ) is an area of the brain where the temporal and parietal lobes meet, at the posterior end of the lateral sulcus. The TPJ incorporates information from the thalamus and the limbic system as well as from the visual, auditory, and somatosensory systems. The TPJ also integrates information from both the external environment as well as from within the body. The TPJ is responsible for collecting all of this information and then processing it.

In neuroscience, the default mode network (DMN), also known as the default network, default state network, or anatomically the medial frontoparietal network (M-FPN), is a large-scale brain network primarily composed of the dorsal medial prefrontal cortex, posterior cingulate cortex, precuneus and angular gyrus. It is best known for being active when a person is not focused on the outside world and the brain is at wakeful rest, such as during daydreaming and mind-wandering. It can also be active during detailed thoughts related to external task performance. Other times that the DMN is active include when the individual is thinking about others, thinking about themselves, remembering the past, and planning for the future.

Resting state fMRI is a method of functional magnetic resonance imaging (fMRI) that is used in brain mapping to evaluate regional interactions that occur in a resting or task-negative state, when an explicit task is not being performed. A number of resting-state brain networks have been identified, one of which is the default mode network. These brain networks are observed through changes in blood flow in the brain which creates what is referred to as a blood-oxygen-level dependent (BOLD) signal that can be measured using fMRI.
The dorsal nexus is an area within the dorsal medial prefrontal cortex that serves as an intersection point for multiple brain networks. Research suggests it plays a role in the maintenance and manipulation of information, as well as supporting the control of cognitive functions such as behavior, memory, and conflict resolution. Abnormally increased connectivity between these networks through the dorsal nexus has been associated with certain types of depression. The activity generated by this abnormally high level of connectivity during a depressive state can be identified through magnetic resonance imaging (MRI) and positron emission tomography (PET).
Amplitude of Low Frequency Fluctuations (ALFF) and fractional Amplitude of Low Frequency Fluctuations (f/ALFF) are neuroimaging methods used to measure spontaneous fluctuations in BOLD-fMRI signal intensity for a given region in the resting brain. Electrophysiological studies suggest that low-frequency oscillations arise from spontaneous neuronal activity. Though ALFFs have been researched extensively in fMRI based theoretical models of brain function, their actual significance is still unknown.

The salience network (SN), also known anatomically as the midcingulo-insular network (M-CIN) or ventral attention network, is a large scale network of the human brain that is primarily composed of the anterior insula (AI) and dorsal anterior cingulate cortex (dACC). It is involved in detecting and filtering salient stimuli, as well as in recruiting relevant functional networks. Together with its interconnected brain networks, the SN contributes to a variety of complex functions, including communication, social behavior, and self-awareness through the integration of sensory, emotional, and cognitive information.
Social cognitive neuroscience is the scientific study of the biological processes underpinning social cognition. Specifically, it uses the tools of neuroscience to study "the mental mechanisms that create, frame, regulate, and respond to our experience of the social world". Social cognitive neuroscience uses the epistemological foundations of cognitive neuroscience, and is closely related to social neuroscience. Social cognitive neuroscience employs human neuroimaging, typically using functional magnetic resonance imaging (fMRI). Human brain stimulation techniques such as transcranial magnetic stimulation and transcranial direct-current stimulation are also used. In nonhuman animals, direct electrophysiological recordings and electrical stimulation of single cells and neuronal populations are utilized for investigating lower-level social cognitive processes.
Network neuroscience is an approach to understanding the structure and function of the human brain through an approach of network science, through the paradigm of graph theory. A network is a connection of many brain regions that interact with each other to give rise to a particular function. Network Neuroscience is a broad field that studies the brain in an integrative way by recording, analyzing, and mapping the brain in various ways. The field studies the brain at multiple scales of analysis to ultimately explain brain systems, behavior, and dysfunction of behavior in psychiatric and neurological diseases. Network neuroscience provides an important theoretical base for understanding neurobiological systems at multiple scales of analysis.

The frontoparietal network (FPN), generally also known as the central executive network (CEN) or, more specifically, the lateral frontoparietal network (L-FPN), is a large-scale brain network primarily composed of the dorsolateral prefrontal cortex and posterior parietal cortex, around the intraparietal sulcus. It is involved in sustained attention, complex problem-solving and working memory.
Functional MRI imaging methods have allowed researchers to combine neurocognitive testing with structural neuroanatomical measures, take into consideration both cognitive and affective paradigms, and subsequently create computer-aided diagnosis techniques and algorithms. Functional MRI has several benefits, such as its non-invasive quality, relatively high spatial resolution, and decent temporal resolution. One particular method used in recent research is resting-state functional magnetic resonance imaging, rs-fMRI. fMRI imaging has been applied to numerous behavioral studies for schizophrenia, the findings of which have hinted toward potential brain regions that govern key characteristics in cognition and affect.















