
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R−O−R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

In organic chemistry, a ketone is a functional group with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.

Astrochemistry is the study of the abundance and reactions of molecules in the universe, and their interaction with radiation. The discipline is an overlap of astronomy and chemistry. The word "astrochemistry" may be applied to both the Solar System and the interstellar medium. The study of the abundance of elements and isotope ratios in Solar System objects, such as meteorites, is also called cosmochemistry, while the study of interstellar atoms and molecules and their interaction with radiation is sometimes called molecular astrophysics. The formation, atomic and chemical composition, evolution and fate of molecular gas clouds is of special interest, because it is from these clouds that solar systems form.

In organic chemistry, an acetal is a functional group with the connectivity R2C(OR')2. Here, the R groups can be organic fragments or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other or not. Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry.

The hydroxyl radical is the diatomic molecule •
OH. The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also important in the field of radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments.

Vinyl alcohol, also called ethenol or ethylenol, is the simplest enol. With the formula CH2CHOH, it is a labile compound that converts to acetaldehyde immediately upon isolation near room temperature. It is not a practical precursor to any compound.
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds are cleaved with ozone. Multiple carbon–carbon bond are replaced by carbonyl groups, such as aldehydes, ketones, and carboxylic acids. The reaction is predominantly applied to alkenes, but alkynes and azo compounds are also susceptible to cleavage. The outcome of the reaction depends on the type of multiple bond being oxidized and the work-up conditions.
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction in which a reactant is subjected to successive chemical reactions in just one reactor. This is much desired by chemists because avoiding a lengthy separation process and purification of the intermediate chemical compounds can save time and resources while increasing chemical yield.
2,4-Dinitrophenylhydrazine (2,4-DNPH or DNPH) is the organic compound C6H3(NO2)2NHNH2. DNPH is a red to orange solid. It is a substituted hydrazine. The solid is relatively sensitive to shock and friction. For this reason DNPH is usually handled as a wet powder. DNPH is a precursor to the drug Sivifene.

Glycolaldehyde is the organic compound with the formula HOCH2−CHO. It is the smallest possible molecule that contains both an aldehyde group and a hydroxyl group. It is a highly reactive molecule that occurs both in the biosphere and in the interstellar medium. It is normally supplied as a white solid. Although it conforms to the general formula for carbohydrates, Cn(H2O)n, it is not generally considered to be a saccharide.
The ethynyl radical (systematically named λ3-ethyne and hydridodicarbon(C—C)) is an organic compound with the chemical formula C≡CH (also written [CCH] or C
2H). It is a simple molecule that does not occur naturally on Earth but is abundant in the interstellar medium. It was first observed by electron spin resonance isolated in a solid argon matrix at liquid helium temperatures in 1963 by Cochran and coworkers at the Johns Hopkins Applied Physics Laboratory. It was first observed in the gas phase by Tucker and coworkers in November 1973 toward the Orion Nebula, using the NRAO 11-meter radio telescope. It has since been detected in a large variety of interstellar environments, including dense molecular clouds, bok globules, star forming regions, the shells around carbon-rich evolved stars, and even in other galaxies.
In organic chemistry, cyanopolyynes are a family of organic compounds with the chemical formula HCnN (n = 3,5,7,…) and the structural formula H−[C≡C−]nC≡N (n = 1,2,3,…). Structurally, they are polyynes with a cyano group (−C≡N) covalently bonded to one of the terminal acetylene units (H−C≡C).

The Quelet reaction is an organic coupling reaction in which a phenolic ether reacts with an aliphatic aldehyde to generate an α-chloroalkyl derivative. The Quelet reaction is an example of a larger class of reaction, electrophilic aromatic substitution. The reaction is named after its creator R. Quelet, who first reported the reaction in 1932, and is similar to the Blanc chloromethylation process.
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is the 3-carbon aldehyde. It is a colourless, flammable liquid with a slightly fruity odour. It is produced on a large scale industrially.
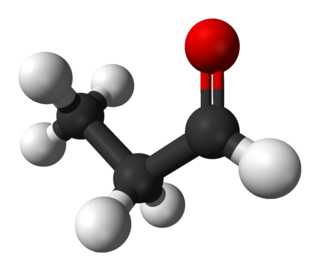
Nitroethylene (also known as nitroethene) is a liquid organic compound with the formula C2H3NO2. It is the simplest nitroalkene, which are unsaturated carbon chains with at least one double bond and a NO2 functional group. Nitroethylene serves as a useful intermediate in the production of various other chemicals.

3-Dimethylaminoacrolein is an organic compound with the formula Me2NC(H)=CHCHO. It is a pale yellow water-soluble liquid. The compound has a number of useful and unusual properties, e.g. it "causes a reversal of the hypnotic effect of morphine in mice" and has a "stimulating effect in humans".
3,9-Divinyl-2,4,8,10-tetraoxaspiro[5.5]undecane (DVTOSU) is a bicyclic organic molecule having a central quaternary carbon atom with which two alicyclic rings are linked, each comprising five atoms. DVTOSU is a diallyl acetal and the precursor for the isomeric ketene acetal monomer 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane (DETOSU) which is a building block for polyorthoesters.

Iodine azide is an explosive inorganic compound, which in ordinary conditions is a yellow solid. Formally, it is an inter-pseudohalogen.

α,β-Unsaturated carbonyl compounds are organic compounds with the general structure (O=CR)−Cα=Cβ-R. Such compounds include enones and enals. In these compounds the carbonyl group is conjugated with an alkene. Unlike the case for carbonyls without a flanking alkene group, α,β-unsaturated carbonyl compounds are susceptible to attack by nucleophiles at the β-carbon. This pattern of reactivity is called vinylogous. Examples of unsaturated carbonyls are acrolein (propenal), mesityl oxide, acrylic acid, and maleic acid. Unsaturated carbonyls can be prepared in the laboratory in an aldol reaction and in the Perkin reaction.

















