
Arsine (IUPAC name: arsane) is an inorganic compound with the formula AsH3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in the semiconductor industry and for the synthesis of organoarsenic compounds. The term arsine is commonly used to describe a class of organoarsenic compounds of the formula AsH3−xRx, where R = aryl or alkyl. For example, As(C6H5)3, called triphenylarsine, is referred to as "an arsine".

Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.
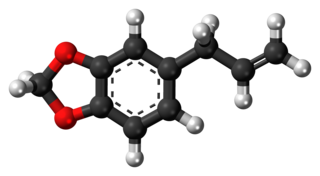
Safrole is an organic compound with the formula CH2O2C6H3CH2CH=CH2. It is a colorless oily liquid, although impure samples can appear yellow. A member of the phenylpropanoid family of natural products, it is found in sassafras plants, among others. Small amounts are found in a wide variety of plants, where it functions as a natural antifeedant. Ocotea pretiosa, which grows in Brazil, and Sassafras albidum, which grows in eastern North America, are the main natural sources of safrole. It has a characteristic "sweet-shop" aroma.

Myristicin is a naturally occurring compound found in common herbs and spices, such as nutmeg. It is an insecticide, and has been shown to enhance the effectiveness of other insecticides.

Chloroacetic acid, industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula ClCH2CO2H. This carboxylic acid is a useful building block in organic synthesis. It is a colorless solid. Related compounds are dichloroacetic acid and trichloroacetic acid.

Carbofuran is a carbamate pesticide, widely used around the world to control insects on a wide variety of field crops, including potatoes, corn and soybeans. It is a systemic insecticide, which means that the plant absorbs it through the roots, and from there the plant distributes it throughout its organs where insecticidal concentrations are attained. Carbofuran also has contact activity against pests. It is one of the most toxic pesticides still in use.

Nitrobenzene is an aromatic nitro compound and the simplest of the nitrobenzenes, with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents.

VG is a "V-series" nerve agent chemically similar to the better-known VX nerve agent. Tetram is the common Russian name for the substance. Amiton was the trade name for the substance when it was marketed as an insecticide by ICI in the mid-1950s.

Paul Hermann Müller, also known as Pauly Mueller, was a Swiss chemist who received the 1948 Nobel prize in Physiology or Medicine for his 1939 discovery of insecticidal qualities and use of DDT in the control of vector diseases such as malaria and yellow fever.

Hans Fischer was a German organic chemist and the recipient of the 1930 Nobel Prize for Chemistry "for his researches into the constitution of haemin and chlorophyll and especially for his synthesis of haemin."

Aldicarb is a carbamate insecticide which is the active substance in the pesticide Temik. It is effective against thrips, aphids, spider mites, lygus, fleahoppers, and leafminers, but is primarily used as a nematicide. Aldicarb is a cholinesterase inhibitor which prevents the breakdown of acetylcholine in the synapse. Aldicarb is considered "extremely hazardous" by the EPA and World Health Organization and has been banned in more than 100 countries. In case of severe poisoning, the victim dies of respiratory failure.

Vinyl acetate is an organic compound with the formula CH3CO2CH=CH2. This colorless liquid is the precursor to polyvinyl acetate, ethene-vinyl acetate copolymers, polyvinyl alcohol, and other important industrial polymers.
2-Chloroethanol (also called ethylene chlorohydrin or glycol chlorohydrin) is an organic chemical compound with the chemical formula HOCH2CH2Cl and the simplest beta-halohydrin (chlorohydrin). This colorless liquid has a pleasant ether-like odor. It is miscible with water. The molecule is bifunctional, consisting of both an alkyl chloride and an alcohol functional group.
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.
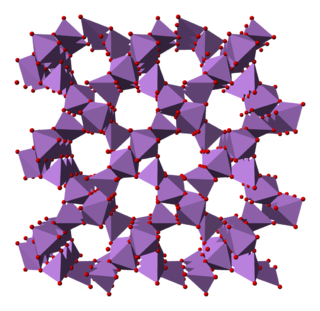
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All inorganic arsenic compounds are highly toxic and thus find only limited commercial applications.

Arsenic trichloride is an inorganic compound with the formula AsCl3, also known as arsenous chloride or butter of arsenic. This poisonous oil is colourless, although impure samples may appear yellow. It is an intermediate in the manufacture of organoarsenic compounds.

Cyanuric fluoride or 2,4,6-trifluoro-1,3,5-triazine is a chemical compound with the formula (CNF)3. It is a colourless, pungent liquid. It has been used as a precursor for fibre-reactive dyes, as a specific reagent for tyrosine residues in enzymes, and as a fluorinating agent.
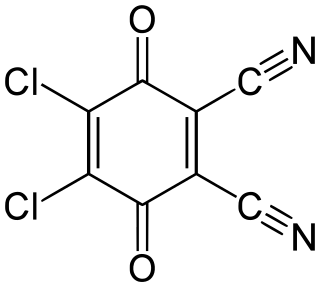
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ) is the chemical reagent with formula C6Cl2(CN)2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols, and steroid ketones. DDQ decomposes in water, but is stable in aqueous mineral acid.
The total synthesis of the complex biomolecule vitamin B12 was accomplished in two different approaches by the collaborating research groups of Robert Burns Woodward at Harvard and Albert Eschenmoser at ETH in 1972. The accomplishment required the effort of no less than 91 postdoctoral researchers (Harvard: 77, ETH: 14), and 12 Ph.D. students (at ETH) from 19 different nations over a period of almost 12 years. The synthesis project induced and involved a major change of paradigm in the field of natural product synthesis.

Methyl thiocyanate is an organic compound with the formula CH3SCN. The simplest member of the organic thiocyanates, it is a colourless liquid with an onion-like odor. It is produced by the methylation of thiocyanate salts. The compound is a precursor to the more useful isomer methyl isothiocyanate (CH3NCS).





















