
The rhinovirus is the most common viral infectious agent in humans and is the predominant cause of the common cold. Rhinovirus infection proliferates in temperatures of 33–35 °C (91–95 °F), the temperatures found in the nose. Rhinoviruses belong to the genus Enterovirus in the family Picornaviridae.

Parvoviruses are a family of animal viruses that constitute the family Parvoviridae. They have linear, single-stranded DNA (ssDNA) genomes that typically contain two genes encoding for a replication initiator protein, called NS1, and the protein the viral capsid is made of. The coding portion of the genome is flanked by telomeres at each end that form into hairpin loops that are important during replication. Parvovirus virions are small compared to most viruses, at 23–28 nanometers in diameter, and contain the genome enclosed in an icosahedral capsid that has a rugged surface.

Hepadnaviridae is a family of viruses. Humans, apes, and birds serve as natural hosts. There are currently 18 species in this family, divided among 5 genera. Its best-known member is hepatitis B virus. Diseases associated with this family include: liver infections, such as hepatitis, hepatocellular carcinomas, and cirrhosis. It is the sole accepted family in the order Blubervirales.

Adenoviruses are medium-sized, nonenveloped viruses with an icosahedral nucleocapsid containing a double-stranded DNA genome. Their name derives from their initial isolation from human adenoids in 1953.

The mumps virus (MuV) is the virus that causes mumps. MuV contains a single-stranded, negative-sense genome made of ribonucleic acid (RNA). Its genome is about 15,000 nucleotides in length and contains seven genes that encode nine proteins. The genome is encased by a capsid that is in turn surrounded by a viral envelope. MuV particles, called virions, are pleomorphic in shape and vary in size from 100 to 600 nanometers in diameter. One serotype and twelve genotypes that vary in their geographic distribution are recognized. Humans are the only natural host of the mumps virus.

Geminiviridae is a family of plant viruses that encode their genetic information on a circular genome of single-stranded (ss) DNA. There are 520 species in this family, assigned to 14 genera. Diseases associated with this family include: bright yellow mosaic, yellow mosaic, yellow mottle, leaf curling, stunting, streaks, reduced yields. They have single-stranded circular DNA genomes encoding genes that diverge in both directions from a virion strand origin of replication. According to the Baltimore classification they are considered class II viruses. It is the largest known family of single stranded DNA viruses.

Adeno-associated viruses (AAV) are small viruses that infect humans and some other primate species. They belong to the genus Dependoparvovirus, which in turn belongs to the family Parvoviridae. They are small replication-defective, nonenveloped viruses and have linear single-stranded DNA (ssDNA) genome of approximately 4.8 kilobases (kb).
Lentivirus is a genus of retroviruses that cause chronic and deadly diseases characterized by long incubation periods, in humans and other mammalian species. The genus includes the human immunodeficiency virus (HIV), which causes AIDS. Lentiviruses are distributed worldwide, and are known to be hosted in apes, cows, goats, horses, cats, and sheep as well as several other mammals.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word ἕρπειν, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. As of 2020, 115 species are recognized, all but one of which are in one of the three subfamilies. Herpesviruses can cause both latent and lytic infections.

Dependoparvovirus is a genus in the subfamily Parvovirinae of the virus family Parvoviridae; they are Group II viruses according to the Baltimore classification. Some dependoparvoviruses are also known as adeno-associated viruses because they cannot replicate productively in their host cell without the cell being coinfected by a helper virus such as an adenovirus, a herpesvirus, or a vaccinia virus.
Kobuvirus is a genus of viruses in the order Picornavirales, in the family Picornaviridae. Humans and cattle serve as natural hosts. There are six species in this genus. Diseases associated with this genus include: gastroenteritis. The genus was named because of the virus particles' lumpy appearance by electron microscopy; "kobu" means "knob" in Japanese.
Erbovirus is a genus of viruses in the order Picornavirales, in the family Picornaviridae. Horses serve as natural hosts. There is only one species in this genus: Erbovirus A. Diseases associated with this genus include: upper respiratory tract disease with viremia and fecal shedding. Viruses belonging to the genus Erbovirus have been isolated in horses with acute upper febrile respiratory disease. The structure of the Erbovirus virion is icosahedral, having a diameter of 27–30 nm.
Vesivirus is a genus of viruses, in the family Caliciviridae. Swine, sea mammals, and felines serve as natural hosts. There are two species in this genus. Diseases associated with this genus include: respiratory disease, Feline calicivirus (FCV); conjunctivitis, and respiratory disease.
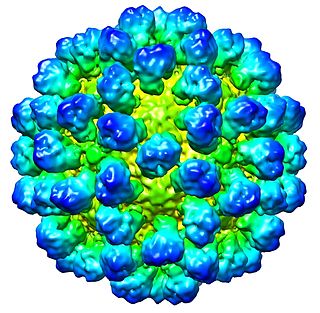
Lagovirus is a genus of viruses, in the family Caliciviridae. Lagomorphs serve as natural hosts. There are two species in this genus. Diseases associated with this genus include: necrotizing hepatitis leading to fatal hemorrhages.
Mason-Pfizer monkey virus (M-PMV), formerly Simian retrovirus (SRV), is a species of retroviruses that usually infect and cause a fatal immune deficiency in Asian macaques. The ssRNA virus appears sporadically in mammary carcinoma of captive macaques at breeding facilities which expected as the natural host, but the prevalence of this virus in feral macaques remains unknown. M-PMV was transmitted naturally by virus-containing body fluids, via biting, scratching, grooming, and fighting. Cross contaminated instruments or equipment (fomite) can also spread this virus among animals.
Adenovirus genomes are linear, non-segmented double-stranded (ds) DNA molecules that are typically 26-46 Kbp long, containing 23-46 protein-coding genes. The example used for the following description is Human adenovirus E, a mastadenovirus with a 36 Kbp genome containing 38 protein-coding genes. While the precise number and identity of genes varies among adenoviruses, the basic principles of genome organization and the functions of most of the genes described in this article are shared among all adenoviruses.

Pneumoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Humans, cattle, and rodents serve as natural hosts. Respiratory tract infections are associated with member viruses such as human respiratory syncytial virus. There are five species in the family which are divided between the genera Metapneumovirus and Orthopneumovirus. The family used to be considered as a sub-family of Paramyxoviridae, but has been reclassified as of 2016.

Avian metaavulavirus 2, formerly Avian paramyxovirus 2, is a species of virus belonging to the family Paramyxoviridae and genus Metaavulavirus. The virus is a negative strand RNA virus containing a monopartite genome. Avian metaavulavirus 2 is one of nine species belonging to the genus Metaavulavirus. The most common serotype of Avulavirinae is serotype 1, the cause of Newcastle disease (ND). Avian metaavulavirus 2 has been known to cause disease, specifically mild respiratory infections in domestic poultry, including turkeys and chickens, and has many economic effects on egg production and poultry industries. The virus was first isolated from a strain in Yucaipa, California in 1956. Since then, other isolates of the virus have been isolated worldwide.
Mammalian orthoreovirus (MRV) is a double-stranded RNA virus. It is a part of the family Reoviridae, as well as the subfamily Spinareovirinae. As seen in the name, the Mammalian Ortheoreovirus infects numerous mammalian species and vertebrates which serve as natural hosts. Some diseases that occur as a result of this virus or are associated with this virus include mild upper respiratory illness, and gastrointestinal illness. Examples of these are: upper respiratory tract syndromes, gastroenteritis, biliary atresia, obstructive hydrocephalus, jaundice, alopecia, conjunctivitis, and ‘oily hair’ associated with steatorrhea.

Astroviridae is a family of non-enveloped ssRNA viruses that cause infections in different animals. The family name is derived from the Greek word astron ("star") referring to the star-like appearance of spikes projecting from the surface of these small unenveloped viruses. Astroviruses were initially identified in humans but have since been isolated from other mammals and birds. This family of viruses consists of two genera, Avastrovirus (AAstV) and Mamastrovirus (MAstV). Astroviruses most frequently cause infection of the gastrointestinal tract but in some animals they may result in encephalitis, hepatitis (avian) and nephritis (avian).












