Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the binding of Ca2+ is required for the activation of calmodulin. Once bound to Ca2+, calmodulin acts as part of a calcium signal transduction pathway by modifying its interactions with various target proteins such as kinases or phosphatases.

Calbindins are three different calcium-binding proteins: calbindin, calretinin and S100G. They were originally described as vitamin D-dependent calcium-binding proteins in the intestine and kidney of chicks and mammals. They are now classified in different subfamilies as they differ in the number of Ca2+ binding EF hands.

TRPV6 is a membrane calcium (Ca2+) channel protein which is particularly involved in the first step in Ca2+absorption in the intestine.
Calcium-binding proteins are proteins that participate in calcium cell signaling pathways by binding to Ca2+, the calcium ion that plays an important role in many cellular processes. Calcium-binding proteins have specific domains that bind to calcium and are known to be heterogeneous.

p16, is a protein that slows cell division by slowing the progression of the cell cycle from the G1 phase to the S phase, thereby acting as a tumor suppressor. It is encoded by the CDKN2A gene. A deletion in this gene can result in insufficient or non-functional p16, accelerating the cell cycle and resulting in many types of cancer.

The plasma membrane Ca2+ ATPase (PMCA) is a transport protein in the plasma membrane of cells that functions as a calcium pump to remove calcium (Ca2+) from the cell. PMCA function is vital for regulating the amount of Ca2+ within all eukaryotic cells. There is a very large transmembrane electrochemical gradient of Ca2+ driving the entry of the ion into cells, yet it is very important that they maintain low concentrations of Ca2+ for proper cell signalling. Thus, it is necessary for cells to employ ion pumps to remove the Ca2+. The PMCA and the sodium calcium exchanger (NCX) are together the main regulators of intracellular Ca2+ concentrations. Since it transports Ca2+ into the extracellular space, the PMCA is also an important regulator of the calcium concentration in the extracellular space.

Parvalbumin (PV) is a calcium-binding protein with low molecular weight. In humans, it is encoded by the PVALB gene. It is not a member of the albumin family; it is named for its size and its ability to coagulate.

The fatty-acid-binding proteins (FABPs) are a family of transport proteins for fatty acids and other lipophilic substances such as eicosanoids and retinoids. These proteins are thought to facilitate the transfer of fatty acids between extra- and intracellular membranes. Some family members are also believed to transport lipophilic molecules from outer cell membrane to certain intracellular receptors such as PPAR. The FABPs are intracellular carriers that “solubilize” the endocannabinoid anandamide (AEA), transporting AEA to the breakdown by FAAH, and compounds that bind to FABPs block AEA breakdown, raising its level. The cannabinoids are also discovered to bind human FABPs that function as intracellular carriers, as THC and CBD inhibit the cellular uptake and catabolism of AEA by targeting FABPs. Competition for FABPs may in part or wholly explain the increased circulating levels of endocannabinoids reported after consumption of cannabinoids. Levels of fatty-acid-binding protein have been shown to decline with ageing in the mouse brain, possibly contributing to age-associated decline in synaptic activity.

Transient receptor potential cation channel subfamily V member 2 is a protein that in humans is encoded by the TRPV2 gene. TRPV2 is a nonspecific cation channel that is a part of the TRP channel family. This channel allows the cell to communicate with its extracellular environment through the transfer of ions, and responds to noxious temperatures greater than 52 °C. It has a structure similar to that of potassium channels, and has similar functions throughout multiple species; recent research has also shown multiple interactions in the human body.

S100 calcium-binding protein A2 (S100A2) is a protein that in humans is encoded by the S100A2 gene and it is located on chromosome 1q21 with other S100 proteins.

Inositol (1,4,5) trisphosphate 3-kinase (EC 2.7.1.127), abbreviated here as ITP3K, is an enzyme that facilitates a phospho-group transfer from adenosine triphosphate to 1D-myo-inositol 1,4,5-trisphosphate. This enzyme belongs to the family of transferases, specifically those transferring phosphorus-containing groups (phosphotransferases) with an alcohol group as acceptor. The systematic name of this enzyme class is ATP:1D-myo-inositol-1,4,5-trisphosphate 3-phosphotransferase. ITP3K catalyzes the transfer of the gamma-phosphate from ATP to the 3-position of inositol 1,4,5-trisphosphate to form inositol 1,3,4,5-tetrakisphosphate. ITP3K is highly specific for the 1,4,5-isomer of IP3, and it exclusively phosphorylates the 3-OH position, producing Ins(1,3,4,5)P4, also known as inositol tetrakisphosphate or IP4.

Plasma membrane calcium-transporting ATPase 4 is an enzyme that in humans is encoded by the ATP2B4 gene.

Protein Wnt-3a is a protein that in humans is encoded by the WNT3A gene.

Mitochondrial fission 1 protein (FIS1) is a protein that in humans is encoded by the FIS1 gene on chromosome 7. This protein is a component of a mitochondrial complex, the ARCosome, that promotes mitochondrial fission. Its role in mitochondrial fission thus implicates it in the regulation of mitochondrial morphology, the cell cycle, and apoptosis. By extension, the protein is involved in associated diseases, including neurodegenerative diseases and cancers.

Copine-1 is a protein that in humans is encoded by the CPNE1 gene.
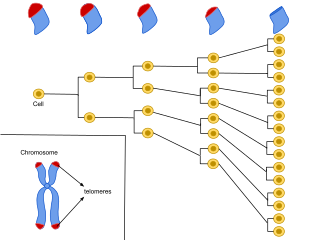
Cellular senescence is a phenomenon characterized by the cessation of cell division. In their experiments during the early 1960s, Leonard Hayflick and Paul Moorhead found that normal human fetal fibroblasts in culture reach a maximum of approximately 50 cell population doublings before becoming senescent. This process is known as "replicative senescence", or the Hayflick limit. Hayflick's discovery of mortal cells paved the path for the discovery and understanding of cellular aging molecular pathways. Cellular senescence can be initiated by a wide variety of stress inducing factors. These stress factors include both environmental and internal damaging events, abnormal cellular growth, oxidative stress, autophagy factors, among many other things.

Protein transport protein Sec16B also known as regucalcin gene promoter region-related protein p117 (RGPR-p117) and leucine zipper transcription regulator 2 (LZTR2) is a protein that in humans is encoded by the SEC16B gene.

Forkhead box protein A2 (FOXA2), also known as hepatocyte nuclear factor 3-beta (HNF-3B), is a transcription factor that plays an important role during development, in mature tissues and, when dysregulated or mutated, also in cancer.

In molecular biology, miR-137 is a short non-coding RNA molecule that functions to regulate the expression levels of other genes by various mechanisms. miR-137 is located on human chromosome 1p22 and has been implicated to act as a tumor suppressor in several cancer types including colorectal cancer, squamous cell carcinoma and melanoma via cell cycle control.

Histidine triad nucleotide binding protein 2 (HINT2) is a mitochondrial protein that in humans is encoded by the HINT2 gene on chromosome 9. This protein is an AMP-lysine hydrolase and phosphoamidase and may contribute to tumor suppression.






















