| Posterior cingulate cortex | |
|---|---|
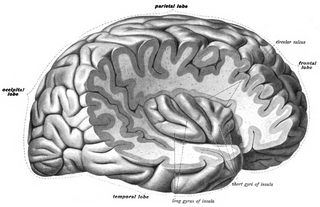
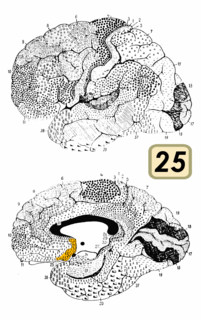
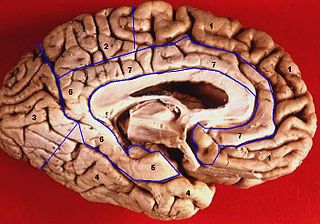
Medial surface of left cerebral hemisphere. | |
Medial surface. (Areas 23 and 31 at center right. The image is reversed from image above.) | |
| Details | |
| Part of | Cingulate gyrus |
| Identifiers | |
| Latin | Cortex cingularis posterior |
| NeuroNames | 162 |
| NeuroLex ID | birnlex_950 |
| FMA | 61924 |

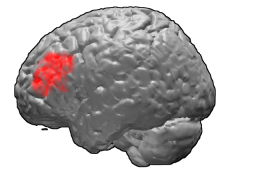
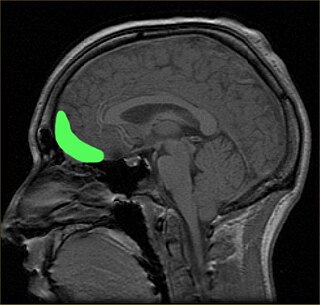
The posterior cingulate cortex (PCC) is the caudal part of the cingulate cortex, located posterior to the anterior cingulate cortex. This is the upper part of the "limbic lobe". The cingulate cortex is made up of an area around the midline of the brain. Surrounding areas include the retrosplenial cortex and the precuneus.

The cingulate cortex is a part of the brain situated in the medial aspect of the cerebral cortex. The cingulate cortex includes the entire cingulate gyrus, which lies immediately above the corpus callosum, and the continuation of this in the cingulate sulcus. The cingulate cortex is usually considered part of the limbic lobe.

The anterior cingulate cortex (ACC) is the frontal part of the cingulate cortex that resembles a "collar" surrounding the frontal part of the corpus callosum. It consists of Brodmann areas 24, 32, and 33.
The limbic lobe is an arc-shaped region of cortex on the medial surface of each cerebral hemisphere of the mammalian brain, consisting of parts of the frontal, parietal and temporal lobes. The term is ambiguous, with some authors including the paraterminal gyrus, the subcallosal area, the cingulate gyrus, the parahippocampal gyrus, the dentate gyrus, the hippocampus and the subiculum; while the Terminologia Anatomica includes the cingulate sulcus, the cingulate gyrus, the isthmus of cingulate gyrus, the fasciolar gyrus, the parahippocampal gyrus, the parahippocampal sulcus, the dentate gyrus, the fimbrodentate sulcus, the fimbria of hippocampus, the collateral sulcus, and the rhinal sulcus, and omits the hippocampus.
Contents
- Anatomy
- Location and boundaries
- Cytoarchitectural organization
- Structural connections
- Function
- Emotion and memory
- Intrinsic control networks
- Meditation
- Disorders
- Alzheimer's disease
- Autism spectrum disorder
- Attention deficit hyperactivity disorder
- Depression
- Schizophrenia
- Traumatic brain injury
- Anxiety disorders
- See also
- References
- External links
Cytoarchitectonically the posterior cingulate cortex is associated with Brodmann areas 23 and 31.

A Brodmann area is a region of the cerebral cortex, in the human or other primate brain, defined by its cytoarchitecture, or histological structure and organization of cells.

Brodmann area 23 (BA23) is a region in the brain corresponding to some portion of the posterior cingulate cortex. It lies between Brodmann area 30 and Brodmann area 31 and is located on the medial wall of the cingulate gyrus between the callosal sulcus and the cingulate sulcus.

Brodmann area 31, also known as dorsal posterior cingulate area 31, is a subdivision of the cytoarchitecturally defined cingulate region of the cerebral cortex. In the human it occupies portions of the posterior cingulate gyrus and medial aspect of the parietal lobe. Approximate boundaries are the cingulate sulcus dorsally and the parieto-occipital sulcus caudally. It partially surrounds the subparietal sulcus, the ventral continuation of the cingulate sulcus in the parietal lobe. Cytoarchitecturally it is bounded rostrally by the ventral anterior cingulate area 24, ventrally by the ventral posterior cingulate area 23, dorsally by the gigantopyramidal area 4 and preparietal area 5 and caudally by the superior parietal area 7 (H) (Brodmann-1909).
The posterior cingulate cortex forms a central node in the default mode network of the brain. It has been shown to communicate with various brain networks simultaneously and is involved in various functions. [1] Along with the precuneus, the posterior cingulate cortex has been implicated as a neural substrate for human awareness in numerous studies of both the anesthesized and vegetative (coma) state. Imaging studies indicate a prominent role for the posterior cingulate cortex in pain and episodic memory retrieval. [2] Increased size of posterior ventral cingulate cortex is related to declines in working memory performance. [3] The posterior cingulate cortex has been strongly implicated as a key part of several intrinsic control networks. [4] [5]

In neuroscience, the default mode network (DMN), also default network, or default state network, is a large scale brain network of interacting brain regions known to have activity highly correlated with each other and distinct from other networks in the brain.