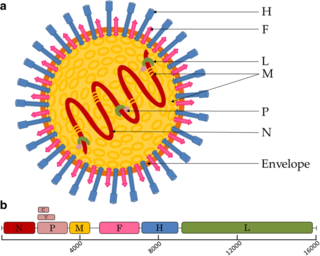
Paramyxoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates serve as natural hosts. Diseases associated with this family include measles, mumps, and respiratory tract infections. The family has four subfamilies, 17 genera, and 78 species, three genera of which are unassigned to a subfamily.

Hepadnaviridae is a family of viruses. Humans, apes, and birds serve as natural hosts. There are currently 18 species in this family, divided among 5 genera. Its best-known member is hepatitis B virus. Diseases associated with this family include: liver infections, such as hepatitis, hepatocellular carcinomas, and cirrhosis. It is the sole accepted family in the order Blubervirales.
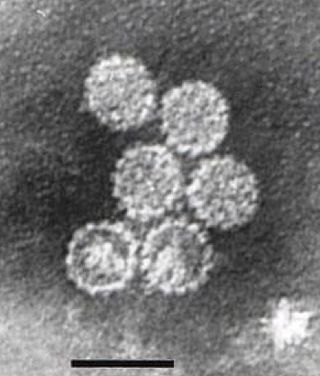
Papillomaviridae is a family of non-enveloped DNA viruses whose members are known as papillomaviruses. Several hundred species of papillomaviruses, traditionally referred to as "types", have been identified infecting all carefully inspected mammals, but also other vertebrates such as birds, snakes, turtles and fish. Infection by most papillomavirus types, depending on the type, is either asymptomatic or causes small benign tumors, known as papillomas or warts. Papillomas caused by some types, however, such as human papillomaviruses 16 and 18, carry a risk of becoming cancerous.

Kaposi's sarcoma-associated herpesvirus (KSHV) is the ninth known human herpesvirus; its formal name according to the International Committee on Taxonomy of Viruses (ICTV) is Human gammaherpesvirus 8, or HHV-8 in short. Like other herpesviruses, its informal names are used interchangeably with its formal ICTV name. This virus causes Kaposi's sarcoma, a cancer commonly occurring in AIDS patients, as well as primary effusion lymphoma, HHV-8-associated multicentric Castleman's disease and KSHV inflammatory cytokine syndrome. It is one of seven currently known human cancer viruses, or oncoviruses. Even after so many years of discovery of KSHV/HHV8, there is no known cure for KSHV associated tumorigenesis.

Tombusviridae is a family of single-stranded positive sense RNA plant viruses. There are three subfamilies, 17 genera, and 95 species in this family. The name is derived from Tomato bushy stunt virus (TBSV).

Tomato bushy stunt virus (TBSV) is a virus of the tombusvirus family. It was first reported in tomatoes in 1935 and primarily affects vegetable crops, though it is not generally considered an economically significant plant pathogen. Depending upon the host, TBSV causes stunting of growth, leaf mottling, and deformed or absent fruit. The virus is likely to be soil-borne in the natural setting, but can also transmitted mechanically, for example through contaminated cutting tools. TBSV has been used as a model system in virology research on the life cycle of plant viruses, particularly in experimental infections of the model host plant Nicotiana benthamiana.
Drosophila X virus (DXV) belongs to the Birnaviridae family of viruses. Birnaviridae currently consists of three genera. The first genus is Entomobirnavirus, which contains DXV. The next genus is Aquabirnavirus, containing infectious pancreatic necrosis virus (IPNV). The last genus is Avibirnavirus, which contains infectious bursal disease virus (IBDV). All of these genera contain homology in three specific areas of their transcripts. The homology comes from the amino and carboxyl regions of preVP2, a small 21-residue-long domain near the carboxyl terminal of VP3, and similar small ORFs sequences.
Luteovirus is a genus of viruses, in the family Tombusviridae. There are 13 species in this genus. Plants serve as natural hosts. The geographical distribution of Luteoviruses is widespread, with the virus primarily infecting plants via transmission by aphid vectors. The virus only replicates within the host cell and not within the vector. The name 'luteovirus' arises from the Latin luteus, which is translated as 'yellow'. Luteovirus was given this name due to the symptomatic yellowing of the plant that occurs as a result of infection.
Strawberry mottle virus (SMV) is a pathogenic plant virus in Secoviridae, a family of plant-infecting picornaviruses. It is not yet assigned to a genus. Virions are isometric, approximately 28 nm in diameter, and contain two RNA strands equal to about 12,600 nucleotides in length. The polyprotein of RNA1 contains regions identified as helicase, protease, RNA-dependent RNA polymerase and a viral genome-linked protein while RNA2 shows similarities to the large coat protein domain of the Satsuma dwarf virus.
Alphasatellites are a single-stranded DNA family of satellite viruses that depend on the presence of another virus to replicate their genomes. As such, they have minimal genomes with very low genomic redundancy. The genome is a single circular single strand DNA molecule. The first alphasatellites were described in 1999 and were associated with cotton leaf curl disease and Ageratum yellow vein disease. As begomoviruses are being characterised at the molecular level an increasing number of alphasatellites are being described.

Fig mosaic emaravirus (FMV) is a segmented, negative sense, single-stranded RNA virus that is determined to be the causal agent of fig mosaic disease (FMD) in fig plants, Ficus carica. It is a member of the genus Emaravirus and order Bunyavirales and is transmitted mainly by the eriophyid mite Aceria ficus. FMV can cause a range of symptoms varying in severity, including leaf chlorosis, deformity, and mosaic or discoloration patterns, as well as premature fruit drop.
Batai orthobunyavirus (BATV) is a RNA virus belonging to order Bunyavirales, genus Orthobunyavirus.
Tilapia tilapinevirus, or Tilapia lake virus (TiLV), is a negative-strand RNA virus that infects both wild and aquacultured populations of tilapia. It is the only species in the monotypic genus Tilapinevirus, which in turn is the only genus in the family Amnoonviridae. Thus far it has been recorded in various regions across Asia, Africa, and South America. The virus was first discovered and identified in 2014 when the Sea of Galilee in Israel experienced a major noticeable decline in tilapia catch quantities.

Agnoprotein is a protein expressed by some members of the polyomavirus family from a gene called the agnogene. Polyomaviruses in which it occurs include two human polyomaviruses associated with disease, BK virus and JC virus, as well as the simian polyomavirus SV40.

RNA silencing suppressor p19 is a protein expressed from the ORF4 gene in the genome of tombusviruses. These viruses are positive-sense single-stranded RNA viruses that infect plant cells, in which RNA silencing forms a widespread and robust antiviral defense system. The p19 protein serves as a counter-defense strategy, specifically binding the 19- to 21-nucleotide double-stranded RNAs that function as small interfering RNA (siRNA) in the RNA silencing system. By sequestering siRNA, p19 suppresses RNA silencing and promotes viral proliferation. The p19 protein is considered a significant virulence factor and a component of an evolutionary arms race between plants and their pathogens.
Triatoma virus (TrV) is a virus belonging to the insect virus family Dicistroviridae. Within this family, there are currently 3 genera and 15 species of virus. Triatoma virus belongs to the genus Cripavirus. It is non-enveloped and its genetic material is positive-sense, single-stranded RNA. The natural hosts of triatoma virus are invertebrates. TrV is a known pathogen to Triatoma infestans, the major vector of Chagas disease in Argentina which makes triatoma virus a major candidate for biological vector control as opposed to chemical insecticides. Triatoma virus was first discovered in 1984 when a survey of pathogens of triatomes was conducted in the hopes of finding potential biological control methods for T. infestans.

The black queen cell virus (BQCV) is a virus that infects honey bees, specifically Apis mellifera, Apis florea, and Apis dorsata. Infection of the latter two species is more recent and can be attributed to genetic similarity and geographical closeness. It is important to learn about this virus because it is one of the most common bee viruses and bees are the most important pollinators. The agricultural industry depends on the bee's pollination to increase its economic value.
Betaarterivirus suid 2 is a species of enveloped, positive-strand RNA viruses which infect domestic pigs. Members of the species are also known as porcine reproductive and respiratory syndrome virus 2. Member viruses are a type of the porcine reproductive and respiratory syndrome viruses (PRRSV). The two types of PRRSV are distinguished by which genomic cluster they are associated with. Type 1 is associated with a LV cluster. Type 2 is associated with a VR2332 cluster.

Modoc virus (MODV) is a rodent-associated flavivirus. Small and enveloped, MODV contains positive single-stranded RNA. Taxonomically, MODV is part of the Flavivirus genus and Flaviviridae family. The Flavivirus genus includes nearly 80 viruses, both vector-borne and no known vector (NKV) species. Known flavivirus vector-borne viruses include Dengue virus, Yellow Fever virus, tick-borne encephalitis virus, and West Nile virus.










