
Heme, or haem, is a ring-shaped iron-containing molecular component of hemoglobin, which is necessary to bind oxygen in the bloodstream. It is composed of four pyrrole rings with 2 vinyl and 2 propionic acid side chains. Heme is biosynthesized in both the bone marrow and the liver.
Steroid hormone receptors are found in the nucleus, cytosol, and also on the plasma membrane of target cells. They are generally intracellular receptors and initiate signal transduction for steroid hormones which lead to changes in gene expression over a time period of hours to days. The best studied steroid hormone receptors are members of the nuclear receptor subfamily 3 (NR3) that include receptors for estrogen and 3-ketosteroids. In addition to nuclear receptors, several G protein-coupled receptors and ion channels act as cell surface receptors for certain steroid hormones.

Human iron metabolism is the set of chemical reactions that maintain human homeostasis of iron at the systemic and cellular level. Iron is both necessary to the body and potentially toxic. Controlling iron levels in the body is a critically important part of many aspects of human health and disease. Hematologists have been especially interested in systemic iron metabolism, because iron is essential for red blood cells, where most of the human body's iron is contained. Understanding iron metabolism is also important for understanding diseases of iron overload, such as hereditary hemochromatosis, and iron deficiency, such as iron-deficiency anemia.

Thromboxane A synthase 1 , also known as TBXAS1, is a cytochrome P450 enzyme that, in humans, is encoded by the TBXAS1 gene.

The liver X receptor (LXR) is a member of the nuclear receptor family of transcription factors and is closely related to nuclear receptors such as the PPARs, FXR and RXR. Liver X receptors (LXRs) are important regulators of cholesterol, fatty acid, and glucose homeostasis. LXRs were earlier classified as orphan nuclear receptors, however, upon discovery of endogenous oxysterols as ligands they were subsequently deorphanized.

Cytochrome P450 17A1 is an enzyme of the hydroxylase type that in humans is encoded by the CYP17A1 gene on chromosome 10. It is ubiquitously expressed in many tissues and cell types, including the zona reticularis and zona fasciculata of the adrenal cortex as well as gonadal tissues. It has both 17α-hydroxylase and 17,20-lyase activities, and is a key enzyme in the steroidogenic pathway that produces progestins, mineralocorticoids, glucocorticoids, androgens, and estrogens. More specifically, the enzyme acts upon pregnenolone and progesterone to add a hydroxyl (-OH) group at carbon 17 position (C17) of the steroid D ring, or acts upon 17α-hydroxyprogesterone and 17α-hydroxypregnenolone to split the side-chain off the steroid nucleus.

Cholesterol 7 alpha-hydroxylase also known as cholesterol 7-alpha-monooxygenase or cytochrome P450 7A1 (CYP7A1) is an enzyme that in humans is encoded by the CYP7A1 gene which has an important role in cholesterol metabolism. It is a cytochrome P450 enzyme, which belongs to the oxidoreductase class, and converts cholesterol to 7-alpha-hydroxycholesterol, the first and rate limiting step in bile acid synthesis.

Steroid 21-hydroxylase is a protein that in humans is encoded by the CYP21A2 gene. The protein is an enzyme that hydroxylates steroids at the C21 position on the molecule. Naming conventions for enzymes are based on the substrate acted upon and the chemical process performed. Biochemically, this enzyme is involved in the biosynthesis of the adrenal gland hormones aldosterone and cortisol, which are important in blood pressure regulation, sodium homeostasis and blood sugar control. The enzyme converts progesterone and 17α-hydroxyprogesterone into 11-deoxycorticosterone and 11-deoxycortisol, respectively, within metabolic pathways which in humans ultimately lead to aldosterone and cortisol creation—deficiency in the enzyme may cause congenital adrenal hyperplasia.

Cytochrome P450 reductase is a membrane-bound enzyme required for electron transfer from NADPH to cytochrome P450 and other heme proteins including heme oxygenase in the endoplasmic reticulum of the eukaryotic cell.

Lanosterol 14α-demethylase (CYP51A1) is the animal version of a cytochrome P450 enzyme that is involved in the conversion of lanosterol to 4,4-dimethylcholesta-8(9),14,24-trien-3β-ol. The cytochrome P450 isoenzymes are a conserved group of proteins that serve as key players in the metabolism of organic substances and the biosynthesis of important steroids, lipids, and vitamins in eukaryotes. As a member of this family, lanosterol 14α-demethylase is responsible for an essential step in the biosynthesis of sterols. In particular, this protein catalyzes the removal of the C-14α-methyl group from lanosterol. This demethylation step is regarded as the initial checkpoint in the transformation of lanosterol to other sterols that are widely used within the cell.

The sigma-2 receptor (σ2R) is a sigma receptor subtype that has attracted attention due to its involvement in diseases such as neurological diseases, neurodegenerative, neuro-ophthalmic and cancer. It is currently under investigation for its potential diagnostic and therapeutic uses.

Cholesterol 24-hydroxylase, also commonly known as cholesterol 24S-hydroxylase, cholesterol 24-monooxygenase, CYP46, or CYP46A1, is an enzyme that catalyzes the conversion of cholesterol to 24S-hydroxycholesterol. It is responsible for the majority of cholesterol turnover in the human central nervous system. The systematic name of this enzyme class is cholesterol,NADPH:oxygen oxidoreductase (24-hydroxylating).

In enzymology, a sterol 14-demethylase (EC 1.14.13.70) is an enzyme of the cytochrome P450 (CYP) superfamily. It is any member of the CYP51 family. It catalyzes a chemical reaction such as:

Cytochrome P450 3A5 is a protein that in humans is encoded by the CYP3A5 gene.

Cytochrome P450 2J2 (CYP2J2) is a protein that in humans is encoded by the CYP2J2 gene. CYP2J2 is a member of the cytochrome P450 superfamily of enzymes. The enzymes are oxygenases which catalyze many reactions involved in the metabolism of drugs and other xenobiotics) as well as in the synthesis of cholesterol, steroids and other lipids.

25-hydroxycholesterol 7-alpha-hydroxylase also known as oxysterol and steroid 7-alpha-hydroxylase is an enzyme that in humans is encoded by the CYP7B1 gene. This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids.

Krueppel-like factor 9 is a protein that in humans is encoded by the KLF9 gene. Previously known as Basic Transcription Element Binding Protein 1, Klf9 is part of the Sp1 C2H2-type zinc finger family of transcription factors. Several previous studies showed Klf9-related regulation of animal development, including cell differentiation of B cells, keratinocytes, and neurons. Klf9 is also a key transcriptional regulator for uterine endometrial cell proliferation, adhesion, and differentiation, all factors that are essential during the process of pregnancy and are turned off during tumorigenesis.
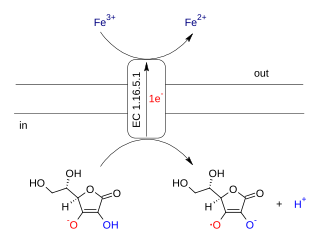
Ascorbate ferrireductase (transmembrane) (EC 1.16.5.1, cytochrome b561) is an enzyme with systematic name Fe(III):ascorbate oxidorectuctase (electron-translocating). This enzyme catalyses the following chemical reaction
Membrane progesterone receptors (mPRs) are a group of cell surface receptors and membrane steroid receptors belonging to the progestin and adipoQ receptor (PAQR) family which bind the endogenous progestogen and neurosteroid progesterone, as well as the neurosteroid allopregnanolone. Unlike the progesterone receptor (PR), a nuclear receptor which mediates its effects via genomic mechanisms, mPRs are cell surface receptors which rapidly alter cell signaling via modulation of intracellular signaling cascades. The mPRs mediate important physiological functions in male and female reproductive tracts, liver, neuroendocrine tissues, and the immune system as well as in breast and ovarian cancer.
Haem or Heme carrier protein 1 (HCP1) was originally identified as mediating heme-Fe transport although it later emerged that it was the SLC46A1 folate transporter.




















