Related Research Articles

A progestogen, also referred to as a progestagen, gestagen, or gestogen, is a type of medication which produces effects similar to those of the natural female sex hormone progesterone in the body. A progestin is a synthetic progestogen. Progestogens are used most commonly in hormonal birth control and menopausal hormone therapy. They can also be used in the treatment of gynecological conditions, to support fertility and pregnancy, to lower sex hormone levels for various purposes, and for other indications. Progestogens are used alone or in combination with estrogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of progestogens include natural or bioidentical progesterone as well as progestins such as medroxyprogesterone acetate and norethisterone.
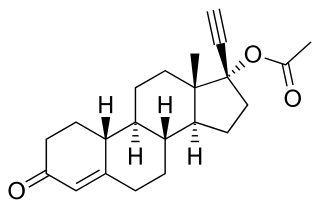
Norethisterone acetate (NETA), also known as norethindrone acetate and sold under the brand name Primolut-Nor among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication available in low-dose and high-dose formulations and is used alone or in combination with an estrogen. It is ingested orally.

Hormonal contraception refers to birth control methods that act on the endocrine system. Almost all methods are composed of steroid hormones, although in India one selective estrogen receptor modulator is marketed as a contraceptive. The original hormonal method—the combined oral contraceptive pill—was first marketed as a contraceptive in 1960. In the ensuing decades, many other delivery methods have been developed, although the oral and injectable methods are by far the most popular. Hormonal contraception is highly effective: when taken on the prescribed schedule, users of steroid hormone methods experience pregnancy rates of less than 1% per year. Perfect-use pregnancy rates for most hormonal contraceptives are usually around the 0.3% rate or less. Currently available methods can only be used by women; the development of a male hormonal contraceptive is an active research area.

Norethisterone, also known as norethindrone and sold under many brand names, is a progestin medication used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication is available in both low-dose and high-dose formulations and both alone and in combination with an estrogen. It is used by mouth or, as norethisterone enanthate, by injection into muscle.

Hydroxyprogesterone caproate, sold under the brand names Proluton and Makena among others, is a medication used to reduce the risk of preterm birth in women pregnant with one baby who have a history of spontaneous preterm birth. In March 2023, the manufacturer, Covis Pharma, agreed to withdraw the drug from the US market. The approvals of Makena and its generics were withdrawn by the US Food and Drug Administration (FDA) in April 2023.
Combined injectable contraceptives (CICs) are a form of hormonal birth control for women. They consist of monthly injections of combined formulations containing an estrogen and a progestin to prevent pregnancy.

Algestone acetophenide, also known more commonly as dihydroxyprogesterone acetophenide (DHPA) and sold under the brand names Perlutal and Topasel among others, is a progestin medication which is used in combination with an estrogen as a form of long-lasting injectable birth control. It has also been used alone, but is no longer available as a standalone medication. DHPA is not active by mouth and is given once a month by injection into muscle.

Medroxyprogesterone acetate (MPA), also known as depot medroxyprogesterone acetate (DMPA) in injectable form and sold under the brand name Depo-Provera among others, is a hormonal medication of the progestin type. It is used as a method of birth control and as a part of menopausal hormone therapy. It is also used to treat endometriosis, abnormal uterine bleeding, paraphilia, and certain types of cancer. The medication is available both alone and in combination with an estrogen. It is taken by mouth, used under the tongue, or by injection into a muscle or fat.

Norethisterone enanthate (NETE), also known as norethindrone enanthate, is a form of hormonal birth control which is used to prevent pregnancy in women. It is used both as a form of progestogen-only injectable birth control and in combined injectable birth control formulations. It may be used following childbirth, miscarriage, or abortion. The failure rate per year in preventing pregnancy for the progestogen-only formulation is 2 per 100 women. Each dose of this form lasts two months with only up to two doses typically recommended.
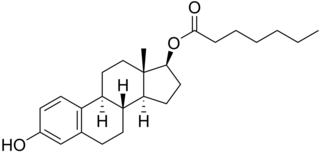
Estradiol enantate, also spelled estradiol enanthate and sold under the brand names Perlutal and Topasel among others, is an estrogen medication which is used in hormonal birth control for women. It is formulated in combination with dihydroxyprogesterone acetophenide, a progestin, and is used specifically as a combined injectable contraceptive. Estradiol enantate is not available for medical use alone. The medication, in combination with DHPA, is given by injection into muscle once a month.

Hydroxyprogesterone acetate (OHPA), sold under the brand name Prodox, is an orally active progestin related to hydroxyprogesterone caproate (OHPC) which has been used in clinical and veterinary medicine. It has reportedly also been used in birth control pills.

Estradiol benzoate butyrate (EBB), sold under the brand names Neolutin N, Redimen, Soluna, and Unijab and formerly known under the developmental code name Unimens, is an estrogen medication which is used in hormonal birth control for women. It is formulated in combination with dihydroxyprogesterone acetophenide, a progestin, and is used specifically as a combined injectable contraceptive. EBB is not available for medical use alone. The medication, in combination with DHPA, is given by injection into muscle once a month.
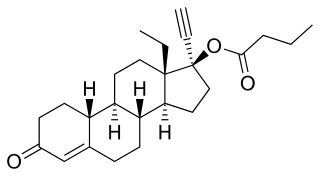
Levonorgestrel butanoate (LNG-B), or levonorgestrel 17β-butanoate, is a steroidal progestin of the 19-nortestosterone group which was developed by the World Health Organization (WHO) in collaboration with the Contraceptive Development Branch (CDB) of the National Institute of Child Health and Human Development as a long-acting injectable contraceptive. It is the C17β butanoate ester of levonorgestrel, and acts as a prodrug of levonorgestrel in the body. The drug is at or beyond the phase III stage of clinical development, but has not been marketed at this time. It was first described in the literature, by the WHO, in 1983, and has been under investigation for potential clinical use since then.

Estradiol valerate/hydroxyprogesterone caproate (EV/OHPC), sold under the brand names Gravibinon and Injectable No. 1 among others, is a combined estrogen and progestogen medication which is used in the treatment of threatened miscarriage and other indications and as a form of combined injectable birth control to prevent pregnancy. It contains estradiol valerate (EV), an estrogen, and hydroxyprogesterone caproate (OHPC), a progestin. The medication is given by injection into muscle once a day to once a month depending on the indication.

Estradiol enantate/algestone acetophenide, also known as estradiol enantate/dihydroxyprogesterone acetophenide (E2-EN/DHPA) and sold under the brand names Perlutal and Topasel among others, is a form of combined injectable birth control which is used to prevent pregnancy. It contains estradiol enantate (E2-EN), an estrogen, and algestone acetophenide, a progestin. The medication is given once a month by injection into muscle.

Estradiol benzoate butyrate/algestone acetophenide, also known as estradiol benzoate butyrate/dihydroxyprogesterone acetophenide (EBB/DHPA) and sold under the brand names Neolutin N, Redimen, Soluna, and Unijab, is a form of combined injectable birth control which is used in Peru and Singapore. It contains estradiol benzoate butyrate (EBB), an estrogen, and algestone acetophenide, a progestin. The medication is given once per month by injection into muscle.

Lynestrenol phenylpropionate (LPP), also known as ethynylestrenol phenylpropionate, is a progestin and a progestogen ester which was developed for potential use as a progestogen-only injectable contraceptive by Organon but was never marketed. It was assessed at doses of 25 to 75 mg in an oil solution once a month by intramuscular injection. LPP was associated with high contraceptive failure at the low dose and with poor cycle control. The medication was found to produce estrogenic effects in the endometrium in women due to transformation into estrogenic metabolites.

Estradiol undecylate/norethisterone enanthate (EU/NETE) is a combination medication of estradiol undecylate (EU), an estrogen, and norethisterone enanthate (NETE), a progestin, which was developed by Schering for potential use as a combined injectable contraceptive in women but was ultimately never marketed. It contained 5 to 10 mg EU and 50 to 70 mg NETE in oil solution and was intended for use by intramuscular injection at regular intervals. Although never commercialized, EU/NETE was found to be effective and well tolerated.

Estradiol valerate/methenmadinone caproate (EV/MMC), known by the tentative brand name Lutofollin, is a combination medication of estradiol valerate (EV), an estrogen, and methenmadinone caproate, a progestin, which was developed for potential use as a once-a-month combined injectable contraceptive but was never marketed. It contained 10 mg EV and 60 mg MMC in 1 mL oil solution and was intended for administration by intramuscular injection once every 4 weeks.
References
- ↑ Singh M, Saxena BB, Singh R, Kaplan J, Ledger WJ (1997). "Contraceptive efficacy of norethindrone encapsulated in injectable biodegradable poly-dl-lactide-co-glycolide microspheres (NET-90): phase III clinical study". Advances in Contraception. 13 (1): 1–11. doi:10.1023/a:1006519027168. PMID 9181181. S2CID 44918557.
In 1957, Karl Junkmann developed norethindrone enanthate (NET-EN or Noristeroir), the first injectable contraceptive which was injected every two months [1].
- 1 2 P. F. A. van Look; Kristian Heggenhougen; Stella R. Quah (January 2011). Sexual and Reproductive Health: A Public Health Perspective. Academic Press. pp. 82–. ISBN 978-0-12-385009-6.
- 1 2 3 4 Nagrath Arun; Malhotra Narendra; Seth Shikha (15 December 2012). Progress in Obstetrics and Gynecology--3. Jaypee Brothers Medical Publishers Pvt. Ltd. pp. 416–. ISBN 978-93-5090-575-3.
- ↑ Mary Lee; Archana Desai (2007). Gibaldi's Drug Delivery Systems in Pharmaceutical Care. ASHP. pp. 328–. ISBN 978-1-58528-136-7.
- ↑ Chaudhuri (1 January 2007). Practice Of Fertility Control: A Comprehensive Manual (7Th ed.). Elsevier India. pp. 154–. ISBN 978-81-312-1150-2.
- 1 2 Hospital, The Royal Women's. "Depo Provera". The Royal Women's Hospital. Retrieved 2024-04-17.
- 1 2 "Oral Contraceptives (Birth Control Pills) and Cancer Risk". www.cancer.gov. March 1, 2018. Retrieved 2024-04-17.
- 1 2 Mokhtar K. Toppozada (1983). "Monthly Injectable Contraceptives". In Alfredo Goldsmith; Mokhtar Toppozada (eds.). Long-Acting Contraception. pp. 93–103. OCLC 35018604.
- ↑ Dr. S. S. Kadam (July 2007). PRINCIPLES OF MEDICINAL CHEMISTRY Vol. - II. Pragati Books Pvt. Ltd. pp. 381–. ISBN 978-81-85790-03-9.
- ↑ Benagiano, G., & Merialdi, M. (2011). Carl Djerassi and the World Health Organisation special programme of research in human reproduction. Journal für Reproduktionsmedizin und Endokrinologie-Journal of Reproductive Medicine and Endocrinology, 8(1), 10-13. http://www.kup.at/kup/pdf/10163.pdf
- ↑ Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.