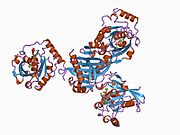
GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved P-loop "G domain", a protein domain common to many GTPases.
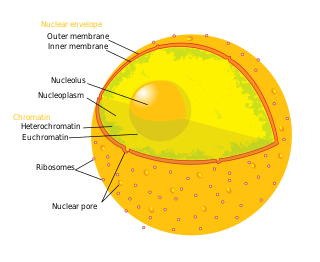
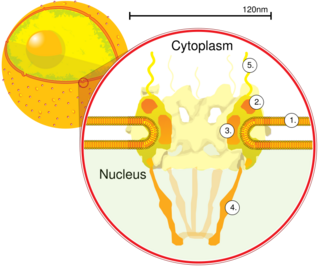
A nuclear pore is a channel as part of the nuclear pore complex (NPC), a large protein complex found in the nuclear envelope in eukaryotic cells, enveloping the cell nucleus containing DNA, which facilitates the selective membrane transport of various molecules across the membrane.
Small GTPases, also known as small G-proteins, are a family of hydrolase enzymes that can bind and hydrolyze guanosine triphosphate (GTP). They are a type of G-protein found in the cytosol that are homologous to the alpha subunit of heterotrimeric G-proteins, but unlike the alpha subunit of G proteins, a small GTPase can function independently as a hydrolase enzyme to bind to and hydrolyze a guanosine triphosphate (GTP) to form guanosine diphosphate (GDP). The best-known members are the Ras GTPases and hence they are sometimes called Ras subfamily GTPases.
A nuclear localization signalorsequence (NLS) is an amino acid sequence that 'tags' a protein for import into the cell nucleus by nuclear transport. Typically, this signal consists of one or more short sequences of positively charged lysines or arginines exposed on the protein surface. Different nuclear localized proteins may share the same NLS. An NLS has the opposite function of a nuclear export signal (NES), which targets proteins out of the nucleus.
Karyopherins are proteins involved in transporting molecules between the cytoplasm and the nucleus of a eukaryotic cell. The inside of the nucleus is called the karyoplasm. Generally, karyopherin-mediated transport occurs through nuclear pores which act as a gateway into and out of the nucleus. Most proteins require karyopherins to traverse the nuclear pore.
Importin is a type of karyopherin that transports protein molecules from the cell's cytoplasm to the nucleus. It does so by binding to specific recognition sequences, called nuclear localization sequences (NLS).
Nuclear transport refers to the mechanisms by which molecules move across the nuclear membrane of a cell. The entry and exit of large molecules from the cell nucleus is tightly controlled by the nuclear pore complexes (NPCs). Although small molecules can enter the nucleus without regulation, macromolecules such as RNA and proteins require association with transport factors known as nuclear transport receptors, like karyopherins called importins to enter the nucleus and exportins to exit.

Guanine nucleotide exchange factors (GEFs) are proteins or protein domains that activate monomeric GTPases by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP). A variety of unrelated structural domains have been shown to exhibit guanine nucleotide exchange activity. Some GEFs can activate multiple GTPases while others are specific to a single GTPase.
Nuclear pore glycoprotein p62 is a protein complex associated with the nuclear envelope. The p62 protein remains associated with the nuclear pore complex-lamina fraction. p62 is synthesized as a soluble cytoplasmic precursor of 61 kDa followed by modification that involve addition of N-acetylglucosamine residues, followed by association with other complex proteins. In humans it is encoded by the NUP62 gene.

Importin subunit beta-1 is a protein that in humans is encoded by the KPNB1 gene.
Nuclear pore complex protein Nup98-Nup96 is a protein that in humans is encoded by the NUP98 gene.

Regulator of chromosome condensation 1, also known as RCC1, Ran guanine nucleotide exchange factor and RanGEF, is the name for a human gene and protein.

Nucleoporin 153 (Nup153) is a protein which in humans is encoded by the NUP153 gene. It is an essential component of the basket of nuclear pore complexes (NPCs) in vertebrates, and required for the anchoring of NPCs. It also acts as the docking site of an importing karyopherin. On the cytoplasmic side of the NPC, Nup358 fulfills an analogous role.

Transportin-1 is a protein that in humans is encoded by the TNPO1 gene.

Ran GTPase-activating protein 1 is an enzyme that in humans is encoded by the RANGAP1 gene.

Ran-specific binding protein 1 is an enzyme that in humans is encoded by the RANBP1 gene.

Importin-7 is a protein that in humans is encoded by the IPO7 gene.

Exportin-T is a protein that in humans is encoded by the XPOT gene.
A nuclear export signal (NES) is a short target peptide containing 4 hydrophobic residues in a protein that targets it for export from the cell nucleus to the cytoplasm through the nuclear pore complex using nuclear transport. It has the opposite effect of a nuclear localization signal, which targets a protein located in the cytoplasm for import to the nucleus. The NES is recognized and bound by exportins.
RanGAP is a protein involved in the transport of other proteins from the cytosol to the nucleus in eukaryotic cells.