
Sulfur (also spelled sulphur in British English) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with the chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature.

Calcium oxide, commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term lime connotes calcium-containing inorganic compounds, in which carbonates, oxides, and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, quicklime specifically applies to the single compound calcium oxide. Calcium oxide that survives processing without reacting in building products, such as cement, is called free lime.
Sulfide (also sulphide in British English ) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.

Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca(OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed with water. Annually, approximately 125 million tons of calcium hydroxide are produced worldwide.

In chemistry, neutralization or neutralisation is a chemical reaction in which acid and a base react with an equivalent quantity of each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends on the acid strength of the reactants.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.
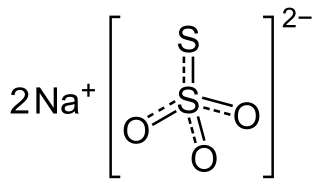
Sodium thiosulfate is an inorganic compound with the formula Na2S2O3·(H2O)x. Typically it is available as the white or colorless pentahydrate, which is a white solid that dissolves well in water. The compound is a reducing agent and a ligand, and these properties underpin its applications.

Flue-gas desulfurization (FGD) is a set of technologies used to remove sulfur dioxide from exhaust flue gases of fossil-fuel power plants, and from the emissions of other sulfur oxide emitting processes such as waste incineration, petroleum refineries, cement and lime kilns.
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionation, such as when a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, is called comproportionation, also known as symproportionation.

The Claus process is the most significant gas desulfurizing process, recovering elemental sulfur from gaseous hydrogen sulfide. First patented in 1883 by the chemist Carl Friedrich Claus, the Claus process has become the industry standard.
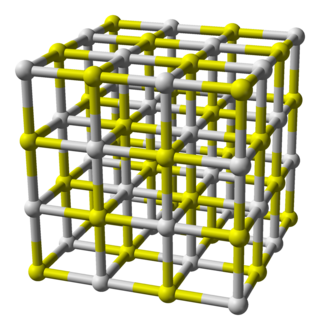
Calcium sulfide is the chemical compound with the formula CaS. This white material crystallizes in cubes like rock salt. CaS has been studied as a component in a process that would recycle gypsum, a product of flue-gas desulfurization. Like many salts containing sulfide ions, CaS typically has an odour of H2S, which results from small amount of this gas formed by hydrolysis of the salt.

The important sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a constituent of many proteins and cofactors, and sulfur compounds can be used as oxidants or reductants in microbial respiration. The global sulfur cycle involves the transformations of sulfur species through different oxidation states, which play an important role in both geological and biological processes. Steps of the sulfur cycle are:

Sodium sulfide is a chemical compound with the formula Na2S, or more commonly its hydrate Na2S·9H2O. Both the anhydrous and the hydrated salts in pure crystalline form are colorless solids, although technical grades of sodium sulfide are generally yellow to brick red owing to the presence of polysulfides and commonly supplied as a crystalline mass, in flake form, or as a fused solid. They are water-soluble, giving strongly alkaline solutions. When exposed to moisture, Na2S immediately hydrates to give sodium hydrosulfide.
Sulfur compounds are chemical compounds formed the element sulfur (S). Common oxidation states of sulfur range from −2 to +6. Sulfur forms stable compounds with all elements except the noble gases.

The dithionite is the oxyanion with the formula [S2O4]2−. It is commonly encountered as the salt sodium dithionite. For historical reasons, it is sometimes called hydrosulfite, but it contains no hydrogen and is not a sulfite. The dianion has a steric number of 4 and trigonal pyramidal geometry.
Magnesium compounds are compounds formed by the element magnesium (Mg). These compounds are important to industry and biology, including magnesium carbonate, magnesium chloride, magnesium citrate, magnesium hydroxide, magnesium oxide, magnesium sulfate, and magnesium sulfate heptahydrate.
A polysulfane is a chemical compound of formula H2Sn, where n > 1. Compounds containing 2 – 8 sulfur atoms have been isolated, longer chain compounds have been detected, but only in solution. H2S2 is colourless, higher members are yellow with the colour increasing with the sulfur content. In the chemical literature the term polysulfanes is sometimes used for compounds containing −(S)n−, e.g. organic polysulfanes R1−(S)n−R2.

Calcium sulfite, or calcium sulphite, is a chemical compound, the calcium salt of sulfite with the formula CaSO3·x(H2O). Two crystalline forms are known, the hemihydrate and the tetrahydrate, respectively CaSO3·½(H2O) and CaSO3·4(H2O). All forms are white solids. It is most notable as the product of flue-gas desulfurization.

Ammonium sulfite is the ammonium salt of sulfurous acid with the chemical formula (NH4)2SO3.
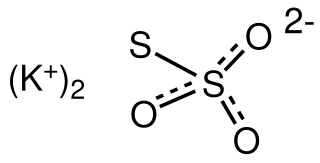
Potassium thiosulfate is an inorganic compound with the formula K2S2O3. This salt can form multiple hydrates, such as the monohydrate, dihydrate, and the pentahydrate, all of which are white or colorless solids. It is used as a fertilizer.













