
Europium(III) chloride is an inorganic compound with the formula EuCl3. The anhydrous compound is a yellow solid. Being hygroscopic it rapidly absorbs water to form a white crystalline hexahydrate, EuCl3·6H2O, which is colourless. The compound is used in research.

Chromium(III) chloride (also called chromic chloride) describes any of several compounds of with the formula CrCl3 · xH2O, where x can be 0, 5, and 6. The anhydrous compound with the formula CrCl3 is a violet solid. The most common form of the trichloride is the dark green "hexahydrate", CrCl3 · 6H2O. Chromium chloride finds uses as catalysts and as precursors to dyes for wool.
Phosphorus trifluoride (formula PF3), is a colorless and odorless gas. It is highly toxic and reacts slowly with water. Its main use is as a ligand in metal complexes. As a ligand, it parallels carbon monoxide in metal carbonyls, and indeed its toxicity is due to its binding with the iron in blood hemoglobin in a similar way to carbon monoxide.
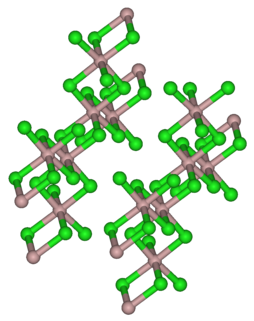
Iridium(III) chloride is the inorganic compound with the formula IrCl3. The anhydrous compound is relatively rare, but the related hydrate is useful for preparing other iridium compounds. The anhydrous salt is a dark green crystalline solid. More commonly encountered is the trihydrate IrCl3(H2O)3.

Bromine pentafluoride, BrF5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorination reagent.

Cobalt(III) fluoride is the inorganic compound with the formula CoF
3. Hydrates are also known. The anhydrous compound is a hygroscopic brown solid. It is used to synthesize organofluorine compounds.

Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF3(H2O)x where x = 0 or 3. They are mainly of interest by researchers, unlike the related iron(III) chlorides. Anhydrous iron(III) fluoride is white, whereas the hydrated forms are light pink.

Ammonium hydrogen fluoride is the inorganic compound with the formula NH4HF2 or NH4F·HF. It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass-etchant and an intermediate in a once-contemplated route to hydrofluoric acid.

Aluminium fluoride (AlF3) is an inorganic compound used primarily in the production of aluminium. This colorless solid can be prepared synthetically but also occurs in nature as minerals rosenbergite and oskarssonite.
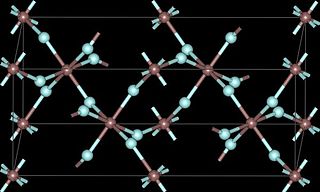
Vanadium(III) fluoride is the chemical compound with the formula VF3. This yellow-green, refractory solid is obtained in a two-step procedure from V2O3. Similar to other transition-metal fluorides (such as MnF2), it exhibits magnetic ordering at low temperatures (e.g. V2F6.4H2O orders below 12 K).

Gallium(III) fluoride (GaF3) is a chemical compound. It is a white solid that melts under pressure above 1000 °C but sublimes around 950 °C. It has the FeF3 structure where the gallium atoms are 6-coordinate. GaF3 can be prepared by reacting F2 or HF with Ga2O3 or by thermal decomposition of (NH4)3GaF6. GaF3 is virtually insoluble in water. Solutions of GaF3 in HF can be evaporated to form the trihydrate, GaF3·3H2O, which on heating gives a hydrated form of GaF2(OH). Gallium(III) fluoride reacts with mineral acids to form hydrofluoric acid.
Antimony trifluoride is the inorganic compound with the formula SbF3. Sometimes called Swart's reagent, is one of two principal fluorides of antimony, the other being SbF5. It appears as a white solid. As well as some industrial applications, it is used as a reagent in inorganic and organofluorine chemistry.

Chromium(III) sulfate usually refers to the inorganic compounds with the formula Cr2(SO4)3.x(H2O), where x can range from 0 to 18. Additionally, ill-defined but commercially important "basic chromium sulfates" are known. These salts are usually either violet or green solids that are soluble in water. It is commonly used in tanning leather.
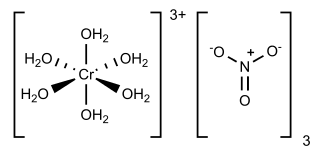
Chromium(III) nitrate describes several inorganic compounds consisting of chromium, nitrate and varying amounts of water. Most common is the dark violet hydrated solid, but an anhydrous green form is also known. Chromium(III) nitrate compounds are of a limited importance commercially, finding some applications in the dyeing industry. It is common in academic laboratories for the synthesis of chromium coordination complexes.
Arsenic trifluoride is a chemical compound of arsenic and fluorine with the chemical formula AsF3. It is a colorless liquid which reacts readily with water.

Bismuth(III) fluoride or bismuth trifluoride is a chemical compound of bismuth and fluorine. The chemical formula is BiF3. It is a grey-white powder melting at 649°C.

Chromium(II) sulfate refers to inorganic compounds with the chemical formula CrSO4·n H2O. Several closely related hydrated salts are known. The pentahydrate is a blue solid that dissolves readily in water. Solutions of chromium(II) are easily oxidized by air to Cr(III) species. Solutions of Cr(II) are used as specialized reducing agents of value in organic synthesis.
Neodymium(III) fluoride is an inorganic chemical compound of neodymium and fluorine with the formula NdF3. It is a purplish pink colored solid with a high melting point. Like other lanthanide fluorides it is highly insoluble in water which allows it to be synthesised from aqueous neodymium nitrate via a reaction with hydrofluoric acid, from which it precipitates as a hydrate:


















