
Antiandrogens, also known as androgen antagonists or testosterone blockers, are a class of drugs that prevent androgens like testosterone and dihydrotestosterone (DHT) from mediating their biological effects in the body. They act by blocking the androgen receptor (AR) and/or inhibiting or suppressing androgen production. They can be thought of as the functional opposites of AR agonists, for instance androgens and anabolic steroids (AAS) like testosterone, DHT, and nandrolone and selective androgen receptor modulators (SARMs) like enobosarm. Antiandrogens are one of three types of sex hormone antagonists, the others being antiestrogens and antiprogestogens.

Bicalutamide, sold under the brand name Casodex among others, is an antiandrogen medication that is primarily used to treat prostate cancer. It is typically used together with a gonadotropin-releasing hormone (GnRH) analogue or surgical removal of the testicles to treat advanced prostate cancer. Bicalutamide may also be used to treat excessive hair growth in women, as a component of feminizing hormone therapy for transgender women, to treat early puberty in boys, and to prevent overly long-lasting erections in men. It is taken by mouth.

Abiraterone acetate, sold under the brand name Zytiga among others, is an antiandrogen medication which is used in the treatment of prostate cancer. It is specifically indicated for use in conjunction with castration and prednisone for the treatment of metastatic castration-resistant prostate cancer (mCRPC) and in the treatment of metastatic high-risk castration-sensitive prostate cancer (mCSPC). It is taken by mouth once per day on an empty stomach. Another brand name for it is Yonsa.

Enzalutamide, sold under the brand name Xtandi, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is indicated for use in conjunction with castration in the treatment of metastatic castration-resistant prostate cancer (mCRPC) and nonmetastatic castration-resistant prostate cancer. It is taken by mouth.
Androgen deprivation therapy (ADT), also called androgen suppression therapy, is an antihormone therapy whose main use is in treating prostate cancer. Prostate cancer cells usually require androgen hormones, such as testosterone, to grow. ADT reduces the levels of androgen hormones, with drugs or surgery, to prevent the prostate cancer cells from growing. The pharmaceutical approaches include antiandrogens and chemical castration.
This article is about the discovery and development of antiandrogens, or androgen receptor (AR) antagonists.

Galeterone is a steroidal antiandrogen which was under development by Tokai Pharmaceuticals for the treatment of prostate cancer. It possesses a unique dual mechanism of action, acting as both an androgen receptor antagonist and a CYP17A1 inhibitor, the latter of which prevents the biosynthesis of androgens. As a CYP17A1 inhibitor, galeterone shows selectivity for 17,20-lyase over 17α-hydroxylase.
EPI-001 was a novel experimental nonsteroidal antiandrogen (NSAA) that was under investigation for the treatment of prostate cancer. The drug was developed by the pharmaceutical company ESSA Pharma Inc for the treatment of castration-resistant prostate cancer (CRPC).

Seviteronel is an experimental cancer medication which is under development by Viamet Pharmaceuticals and Innocrin Pharmaceuticals for the treatment of prostate cancer and breast cancer. It is a nonsteroidal CYP17A1 inhibitor and works by inhibiting the production of androgens and estrogens in the body. As of July 2017, seviteronel is in phase II clinical trials for both prostate cancer and breast cancer. In January 2016, it was designated fast-track status by the United States Food and Drug Administration for prostate cancer. In April 2017, seviteronel received fast-track designation for breast cancer as well.

Apalutamide, sold under the brand name Erleada, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is specifically indicated for use in conjunction with castration in the treatment of non-metastatic castration-resistant prostate cancer (NM-CRPC). It is taken by mouth.

Ralaniten acetate is an experimental nonsteroidal antiandrogen (NSAA) which was developed by ESSA Pharmaceuticals and was under investigation for the treatment of prostate cancer. It was a successor of EPI-001 and targets the N-terminal domain (NTD) of the androgen receptor (AR). This mechanism of action is believed to allow the drug to block signaling from the AR and its splice variants. EPI-506 is a derivative of bisphenol A and a prodrug of ralaniten (EPI-002), one of the four stereoisomers of EPI-001. The drug reached phase I/II prior to the discontinuation of its development. It showed signs of efficacy in the form of prostatic specific antigen (PSA) decreases (4–29%) predominantly at higher doses (≥1,280 mg) in some patients but also caused side effects and was discontinued by its developer in favor of next-generation AR NTD inhibitors with improved potency and tolerability.
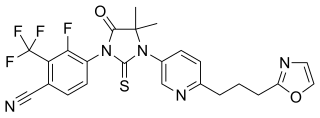
Proxalutamide is a nonsteroidal antiandrogen (NSAA) – specifically, a selective high-affinity silent antagonist of the androgen receptor (AR) – which is under development by Suzhou Kintor Pharmaceuticals for the treatment of prostate cancer. It inhibits AR-mediated gene transcription more potently than bicalutamide and enzalutamide and maintains silent antagonism in castration-resistant prostate cancer (CRPC) cells. It has also been found to downregulate the AR, which could further confer it greater efficacy against CRPC compared to existing NSAAs. Unlike enzalutamide, the drug showed low central nervous system distribution and no induction of seizures in animals. As of 2017, it is in phase II clinical trials for prostate cancer. It is also in preclinical investigation for the treatment of AR-positive breast cancer.

N-Desmethylenzalutamide is a nonsteroidal antiandrogen (NSAA) and the major metabolite of enzalutamide, an NSAA which is used as a hormonal antineoplastic agent in the treatment of metastatic prostate cancer. It has similar activity to that of enzalutamide and, with enzalutamide therapy, circulates at similar concentrations to those of enzalutamide at steady state. N-Desmethylenzalutamide is formed from enzalutamide in the liver by the cytochrome P450 enzymes CYP2C8 and CYP3A4. It has a longer terminal half-life than enzalutamide.

5N-Bicalutamide, or 5-azabicalutamide, is a highly potent nonsteroidal antiandrogen (NSAA) which was discovered in 2016. It is a structural modification of bicalutamide differing it from it only by the replacement of a carbon atom with a nitrogen atom in one of its phenyl rings. Similarly to bicalutamide, the drug acts as a selective antagonist of the androgen receptor (AR). However, unlike bicalutamide, it is a reversible covalent antagonist and stays bound to the receptor for a far longer amount of time. As a result of this difference, 5N-bicalutamide has markedly improved potency relative to bicalutamide, with approximately 150-fold higher affinity for the AR (Ki = 0.15 nM versus 22.3 nM) and about 20-fold greater functional inhibition (IC50 = 15 nM versus 310 nM) of the AR. Future studies of 5N-bicalutamide in normal and mutated prostate cancer cells are planned or underway and it is anticipated that N-bicalutamide may be able to overcome resistance to current antiandrogens that are used in the treatment of prostate cancer.
Comparison of the nonsteroidal antiandrogen (NSAA) bicalutamide with other antiandrogens reveals differences between the medications in terms of efficacy, tolerability, safety, and other parameters. Relative to the other first-generation NSAAs, flutamide and nilutamide, bicalutamide shows improved potency, efficacy, tolerability, and safety, and has largely replaced these medications in clinical practice. Compared to the second-generation NSAAs, enzalutamide and apalutamide, bicalutamide has inferior potency and efficacy but similar tolerability and safety and a lower propensity for drug interactions.

RD-162 is a second-generation nonsteroidal antiandrogen (NSAA) which was developed for the treatment of prostate cancer but was never marketed. It acts as a potent and selective silent antagonist of the androgen receptor (AR). The drug is a diarylthiohydantoin derivative and is closely related to enzalutamide and apalutamide. Both RD-162 and enzalutamide show 5- to 8-fold higher affinity for the AR than the first-generation NSAA bicalutamide and only 2- to 3-fold lower affinity than dihydrotestosterone (DHT), the major endogenous ligand of the receptor in the prostate gland.

RU-59063 is a nonsteroidal androgen or selective androgen receptor modulator (SARM) which was first described in 1994 and was never marketed. It was originally thought to be a potent antiandrogen, but subsequent research found that it actually possesses dose-dependent androgenic activity, albeit with lower efficacy than dihydrotestosterone (DHT). The drug is an N-substituted arylthiohydantoin and was derived from the first-generation nonsteroidal antiandrogen (NSAA) nilutamide. The second-generation NSAAs enzalutamide, RD-162, and apalutamide were derived from RU-59063.













