Related Research Articles

Berkelium is a synthetic chemical element; it has symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium.
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during reactions with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds.
Tridecylic acid, or tridecanoic acid, is the organic compound with the formula CH3(CH2)11CO2H. It is a 13-carbon saturated fatty acid. It is a white solid.

Few compounds of californium have been made and studied. The only californium ion that is stable in aqueous solutions is the californium(III) cation. The other two oxidation states are IV (strong oxidizing agents) and II (strong reducing agents). The element forms a water-soluble chloride, nitrate, perchlorate, and sulfate and is precipitated as a fluoride, oxalate or hydroxide. If problems of availability of the element could be overcome, then CfBr2 and CfI2 would likely be stable.

Berkelium forms a number of chemical compounds, where it normally exists in an oxidation state of +3 or +4, and behaves similarly to its lanthanide analogue, terbium. Like all actinides, berkelium easily dissolves in various aqueous inorganic acids, liberating gaseous hydrogen and converting into the trivalent oxidation state. This trivalent state is the most stable, especially in aqueous solutions, but tetravalent berkelium compounds are also known. The existence of divalent berkelium salts is uncertain and has only been reported in mixed lanthanum chloride-strontium chloride melts. Aqueous solutions of Bk3+ ions are green in most acids. The color of the Bk4+ ions is yellow in hydrochloric acid and orange-yellow in sulfuric acid. Berkelium does not react rapidly with oxygen at room temperature, possibly due to the formation of a protective oxide surface layer; however, it reacts with molten metals, hydrogen, halogens, chalcogens and pnictogens to form various binary compounds. Berkelium can also form several organometallic compounds.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

Bismuth titanate or bismuth titanium oxide is a solid inorganic compound of bismuth, titanium and oxygen with the chemical formula of Bi12TiO20, Bi 4Ti3O12 or Bi2Ti2O7.

Pentamethyltantalum is a homoleptic organotantalum compound. It has a propensity to explode when it is melted. Its discovery was part of a sequence that led to Richard R. Schrock's Nobel Prize winning discovery in olefin metathesis.

Berkelium(III) chloride also known as berkelium trichloride, is a chemical compound with the formula BkCl3. It is a water-soluble green salt with a melting point of 603 °C. This compound forms the hexahydrate, BkCl3·6H2O.

Holmium(III) iodide is an iodide of holmium, with the chemical formula of HoI3. It is used as a component of metal halide lamps.

Ytterbium(II) fluoride is a binary inorganic compound of ytterbium and fluorine with the chemical formula YbF2.

Berkelium(III) iodide is a binary inorganic compound of berkelium and iodine with the chemical formula BkI3.

Berkelium(III) oxide is a binary inorganic compound of berkelium and oxygen with the chemical formula Bk
2O
3.

Iodine dioxide is a binary inorganic compound of iodine and oxygen with the chemical formula IO
2. This compound is one of many iodine oxides.
Einsteinium fluoride is a binary inorganic chemical compound of einsteinium and fluorine with the chemical formula EsF3.
Einsteinium(II) chloride is a binary inorganic chemical compound of einsteinium and chlorine with the chemical formula EsCl2.
Einsteinium(II) bromide is a binary inorganic chemical compound of einsteinium and bromine with the chemical formula EsBr2.
Americium trihydride is a binary inorganic compound of americium and hydrogen with the chemical formula AmH3.
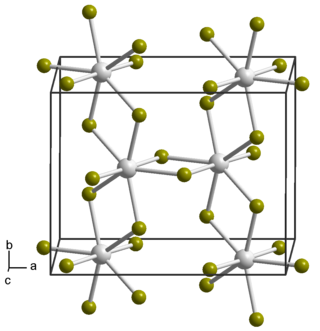
Neptunium tetrabromide is a binary inorganic compound of neptunium metal and bromine with the chemical formula NpBr4.
Thorium triiodide is a binary inorganic compound of thorium metal and iodine with the chemical formula ThI3.
References
- ↑ "WebElements Periodic Table » Berkelium » berkelium oxide". webelements.com. Retrieved 28 January 2024.
- ↑ Yaws, Carl L. (6 January 2015). The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals: Physical Properties for More Than 54,000 Organic and Inorganic Chemical Compounds, Coverage for C1 to C100 Organics and Ac to Zr Inorganics. Gulf Professional Publishing. p. 698. ISBN 978-0-12-801146-1 . Retrieved 28 January 2024.
- ↑ Seaborg, G. T.; Katz, Joseph J.; Morss, L. R. (6 December 2012). The Chemistry of the Actinide Elements: Volume 2. Springer Science & Business Media. p. 1003. ISBN 978-94-009-3155-8 . Retrieved 28 January 2024.
- ↑ Sabry, Fouad (15 October 2022). Americium: Future space missions can be powered for up to 400 years. One Billion Knowledgeable. Retrieved 28 January 2024.