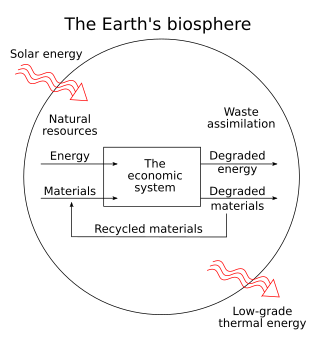
Ecological economics, bioeconomics, ecolonomy, eco-economics, or ecol-econ is both a transdisciplinary and an interdisciplinary field of academic research addressing the interdependence and coevolution of human economies and natural ecosystems, both intertemporally and spatially. By treating the economy as a subsystem of Earth's larger ecosystem, and by emphasizing the preservation of natural capital, the field of ecological economics is differentiated from environmental economics, which is the mainstream economic analysis of the environment. One survey of German economists found that ecological and environmental economics are different schools of economic thought, with ecological economists emphasizing strong sustainability and rejecting the proposition that physical (human-made) capital can substitute for natural capital.
Efficiency is the often measurable ability to avoid wasting materials, energy, efforts, money, and time while performing a task. In a more general sense, it is the ability to do things well, successfully, and without waste.
Howard Thomas Odum, usually cited as H. T. Odum, was an American ecologist. He is known for his pioneering work on ecosystem ecology, and for his provocative proposals for additional laws of thermodynamics, informed by his work on general systems theory.
From a scientific and engineering perspective, second-law based exergy analysis is valuable because it provides a number of benefits over energy analysis alone. These benefits include the basis for determining energy quality, enhancing the understanding of fundamental physical phenomena, and improving design, performance evaluation and optimization efforts. In thermodynamics, the exergy of a system is the maximum useful work that can be produced as the system is brought into equilibrium with its environment by an ideal process. The specification of an 'ideal process' allows the determination of 'maximum work' production. From a conceptual perspective, exergy is the 'ideal' potential of a system to do work or cause a change as it achieves equilibrium with its environment. Exergy is also known as 'availability'. Exergy is non-zero when there is dis-equilibrium between the system and its environment, and exergy is zero when equilibrium is established.
In energy economics and ecological energetics, energy return on investment (EROI), also sometimes called energy returned on energy invested (ERoEI), is the ratio of the amount of usable energy delivered from a particular energy resource to the amount of exergy used to obtain that energy resource.
Primary energy (PE) is the energy found in nature that has not been subjected to any human engineered conversion process. It encompasses energy contained in raw fuels and other forms of energy, including waste, received as input to a system. Primary energy can be non-renewable or renewable.

Ecological engineering uses ecology and engineering to predict, design, construct or restore, and manage ecosystems that integrate "human society with its natural environment for the benefit of both".
Emergy is the amount of energy consumed in direct and indirect transformations to make a product or service. Emergy is a measure of quality differences between different forms of energy. Emergy is an expression of all the energy used in the work processes that generate a product or service in units of one type of energy. Emergy is measured in units of emjoules, a unit referring to the available energy consumed in transformations. Emergy accounts for different forms of energy and resources Each form is generated by transformation processes in nature and each has a different ability to support work in natural and in human systems. The recognition of these quality differences is a key concept.
The following outline is provided as an overview of and topical guide to energy:
"Unified Science" can refer to any of three related strands in contemporary thought.
The energy systems language, also referred to as energese, or energy circuit language, or generic systems symbols, is a modelling language used for composing energy flow diagrams in the field of systems ecology. It was developed by Howard T. Odum and colleagues in the 1950s during studies of the tropical forests funded by the United States Atomic Energy Commission.
In 1996 H.T. Odum defined transformity as,
"the emergy of one type required to make a unit of energy of another type. For example, since 3 coal emjoules (cej) of coal and 1 cej of services are required to generate 1 J of electricity, the coal transformity of electricity is 4 cej/J"

An ecological pyramid is a graphical representation designed to show the biomass or bioproductivity at each trophic level in an ecosystem.

The maximum power principle or Lotka's principle has been proposed as the fourth principle of energetics in open system thermodynamics, where an example of an open system is a biological cell. According to Howard T. Odum, "The maximum power principle can be stated: During self-organization, system designs develop and prevail that maximize power intake, energy transformation, and those uses that reinforce production and efficiency."
David M. Scienceman is an Australian scientist; he changed his name from David Slade by deed poll in 1972. McGhee (1990) wrote that his change of name from Slade to Scienceman was an experiment to create a movement of scientifically aware politicians. In a world dominated by scientific achievements and problems, Slade believed that there should be a political party that represented the scientific point of view.

Energy conversion efficiency (η) is the ratio between the useful output of an energy conversion machine and the input, in energy terms. The input, as well as the useful output may be chemical, electric power, mechanical work, light (radiation), or heat. The resulting value, η (eta), ranges between 0 and 1.
Research concerning the relationship between the thermodynamic quantity entropy and both the origin and evolution of life began around the turn of the 20th century. In 1910, American historian Henry Adams printed and distributed to university libraries and history professors the small volume A Letter to American Teachers of History proposing a theory of history based on the second law of thermodynamics and on the principle of entropy.
Corrado Giannantoni is an Italian nuclear scientist.
Sustainability metrics and indices are measures of sustainability, and attempt to quantify beyond the generic concept. Though there are disagreements among those from different disciplines, these disciplines and international organizations have each offered measures or indicators of how to measure the concept.
The Carnot method is an allocation procedure for dividing up fuel input (primary energy, end energy) in joint production processes that generate two or more energy products in one process (e.g. cogeneration or trigeneration). It is also suited to allocate other streams such as CO2-emissions or variable costs. The potential to provide physical work (exergy) is used as the distribution key. For heat this potential can be assessed the Carnot efficiency. Thus, the Carnot method is a form of an exergetic allocation method. It uses mean heat grid temperatures at the output of the process as a calculation basis. The Carnot method's advantage is that no external reference values are required to allocate the input to the different output streams; only endogenous process parameters are needed. Thus, the allocation results remain unbiased of assumptions or external reference values that are open for discussion.






