
Selective androgen receptor modulators (SARMs) are a class of drugs that selectively activate the androgen receptor in specific tissues, promoting muscle and bone growth while having less effect on male reproductive tissues like the prostate gland.

BMS-564,929 is an investigational selective androgen receptor modulator (SARM) which is being developed by Bristol-Myers Squibb for treatment of the symptoms of age-related decline in androgen levels in men ("andropause"). These symptoms may include depression, loss of muscle mass and strength, reduction in libido and osteoporosis. Treatment with exogenous testosterone is effective in counteracting these symptoms but is associated with a range of side effects, the most serious of which is enlargement of the prostate gland, which can lead to benign prostatic hyperplasia and even prostate cancer. This means there is a clinical need for selective androgen receptor modulators, which produce anabolic effects in some tissues such as muscle and bone, but without stimulating androgen receptors in the prostate.

S-40503 is an investigational selective androgen receptor modulator (SARM) developed by the Japanese company Kaken Pharmaceuticals, which was developed for the treatment of osteoporosis. SARMs are a new class of drugs that produce tissue-specific anabolic effects in some tissues such as muscle and bone, but without stimulating androgen receptors in other tissues such as in the prostate gland, thus avoiding side effects such as benign prostatic hyperplasia which can occur following treatment with unselective androgens like testosterone or anabolic steroids.

LGD-2226 is an investigational selective androgen receptor modulator (SARM), which is being developed for treatment of muscle wasting and osteoporosis.

Enobosarm, also formerly known as ostarine and by the developmental code names GTx-024, MK-2866, and S-22, is a selective androgen receptor modulator (SARM) which is under development for the treatment of androgen receptor-positive breast cancer in women and for improvement of body composition in people taking GLP-1 receptor agonists like semaglutide. It was also under development for a variety of other indications, including treatment of cachexia, Duchenne muscular dystrophy, muscle atrophy or sarcopenia, and stress urinary incontinence, but development for all other uses has been discontinued. Enobosarm was evaluated for the treatment of muscle wasting related to cancer in late-stage clinical trials, and the drug improved lean body mass in these trials, but it was not effective in improving muscle strength. As a result, enobosarm was not approved and development for this use was terminated. Enobosarm is taken by mouth.
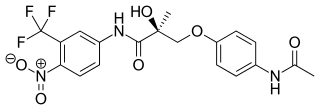
Andarine is a selective androgen receptor modulator (SARM) which was developed by GTX, Inc for the treatment of conditions such as muscle wasting, osteoporosis, and benign prostatic hyperplasia (BPH), using the nonsteroidal antiandrogen bicalutamide as a lead compound. Development of andarine for all indications has been discontinued, in favor of the structurally related and improved compound enobosarm.

S-23 is an investigational selective androgen receptor modulator (SARM) developed by GTX, Inc as a potential male hormonal contraceptive. It binds to the androgen receptor more strongly than older drugs such as andarine with a Ki of 1.7 nM, and in animal studies it showed both a good ratio of anabolic to androgenic effects, and dose-dependent suppression of spermatogenesis with spontaneous recovery after cessation of treatment.

LGD-4033, also known by the developmental code name VK5211 and by the black-market name Ligandrol, is a selective androgen receptor modulator (SARM) which is under development for the treatment of muscle atrophy in people with hip fracture. It was also under development for the treatment of cachexia, hypogonadism, and osteoporosis, but development for these indications was discontinued. LGD-4033 has been reported to dose-dependently improve lean body mass and muscle strength in preliminary clinical trials, but is still being developed and has not been approved for medical use. The drug is taken by mouth.

Vosilasarm, also known by the development codes RAD140 and EP0062 and by the black-market name Testolone or Testalone, is a selective androgen receptor modulator (SARM) which is under development for the treatment of hormone-sensitive breast cancer. It is specifically under development for the treatment of androgen receptor-positive, estrogen receptor-negative, HER2-negative advanced breast cancer. Vosilasarm was also previously under development for the treatment of sarcopenia, osteoporosis, and weight loss due to cancer cachexia, but development for these indications was discontinued. The drug is taken by mouth.
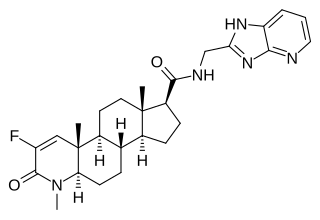
MK-0773, also known as PF-05314882, is a steroidal, orally active selective androgen receptor modulator (SARM) that was under development by Merck and GTx for the treatment of sarcopenia in women and men. Clinical trials for sarcopenia began in late 2007 but the collaboration between Merck and GTx ended in early 2010 and GTx terminated development of MK-0773 shortly thereafter. MK-0773 was developed as a more advanced version of the related compound TFM-4AS-1.
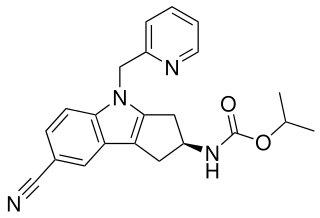
OPK-88004 is a non-steroidal indole derivative which acts as a selective androgen receptor modulator (SARM). It has been investigated by OPKO Health for the treatment of erectile dysfunction and symptoms associated with benign prostate hyperplasia.

GSK2881078 is a drug which acts as a selective androgen receptor modulator (SARM). It was developed for the prevention of muscle wasting and sarcopenia in elderly people.
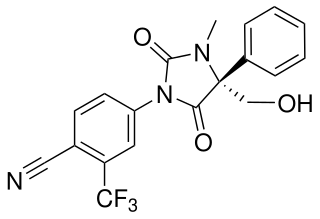
GLPG-0492 (DT-200) is a drug which acts as a selective androgen receptor modulator (SARM). It has been investigated for the treatment of cachexia and muscular dystrophy.

PF-06260414 is a drug which acts as a selective androgen receptor modulator (SARM), and was developed for androgen replacement therapy.

GSK-971086 is an investigational new drug that is a selective androgen receptor modulator (SARM) that was being developed by GlaxoSmithKline (GSK) for the potential treatment of sarcopenia. As a SARM, GSK-971086 was designed to target androgen receptors in specific tissues, potentially offering therapeutic benefits for muscle wasting conditions while minimizing unwanted androgenic side effects. The compound underwent early-stage clinical trials to evaluate its safety, tolerability, and pharmacokinetic profile in human subjects.
Arcarine (ORM-11984) is a selective androgen receptor modulator (SARM) developed by Orion Corporation, a Finnish pharmaceutical company. It belongs to a class of drugs designed to have tissue-selective androgenic effects, potentially offering the benefits of androgens while minimizing unwanted side effects. Arcarine was investigated for the treatment of various conditions, including benign prostatic hyperplasia, hypogonadism, and osteoporosis. The compound reached Phase I clinical trials before development was discontinued. Like other SARMs, Arcarine was developed to potentially provide anabolic effects in muscle and bone tissue while having reduced androgenic effects in other tissues, such as the prostate.

JNJ-37654032 is a selective androgen receptor modulator (SARM) which was developed by Johnson & Johnson for the potential treatment of muscular atrophy but was never marketed.

GTx-027 is a selective androgen receptor modulator (SARM) which was under development for or of potential interest in the treatment of breast cancer and stress urinary incontinence (SUI) but was never marketed. It is taken by mouth.

MK-4541 is a dual selective androgen receptor modulator (SARM) and 5α-reductase inhibitor (5α-RI) which has been of interest for the potential treatment of prostate cancer but has not been marketed at this time. It is intended for use by mouth.

















