
The Antarctic toothfish, also known as the Antarctic cod, is a large, black or brown fish found in very cold (subzero) waters of the Southern Ocean near Antarctica. It is the largest fish in the Southern Ocean, feeding on shrimp and smaller fish, and preyed on by whales, orcas, and seals. It is caught for food and marketed as Chilean sea bass together with its sister species, the more northerly Patagonian toothfish. Often mistakenly called "Antarctic cod", the Antarctic toothfish belongs to the notothen family (Nototheniidae), a family of fish genera that are abundant near Antarctica.

The Patagonian toothfish, also known as Chilean sea bass, mero, and icefish, is a species of notothen found in cold waters between depths of 45 and 3,850 m in the southern Atlantic, Pacific, and Indian Oceans and Southern Ocean on seamounts and continental shelves around most Subantarctic islands.

The Beryciformes are a poorly-understood order of carnivorous ray-finned fishes consisting of 7 families, 30 genera, and 161 species. They feed on small fish and invertebrates. Beyond this, little is known about the biology of most member species because of their nocturnal habits and deepwater habitats. All beryciform species are marine and most live in tropical to temperate, deepwater environments. Most live on the continental shelf and continental slope, with some species being found as deep as 2,000 m (6,600 ft). Some species move closer to the surface at night, while others live entirely in shallow water and are nocturnal, hiding in rock crevices and caves during the day. Several species are mesopelagic and bathypelagic. Beryciformes' bodies are deep and mildly compressed, typically with large eyes that help them see in darker waters. Colors range from red to yellow and brown to black, and sizes range from 8–61 cm (3.1–24.0 in). Member genera include the alfonsinos, squirrelfishes, flashlight fishes, fangtooth fishes, spinyfins, pineconefishes, redfishes, roughies, and slimeheads. A number of member species are caught commercially, including the alfonsino, the splendid alfonsino, and the orange roughy, the latter being much more economically important. Some species have bioluminescent bacteria contained in pockets of skin or in light organs near the eyes, including the anomalopids and monocentrids.

Overfishing is the removal of a species of fish from a body of water at a rate greater than that the species can replenish its population naturally, resulting in the species becoming increasingly underpopulated in that area. Overfishing can occur in water bodies of any sizes, such as ponds, wetlands, rivers, lakes or oceans, and can result in resource depletion, reduced biological growth rates and low biomass levels. Sustained overfishing can lead to critical depensation, where the fish population is no longer able to sustain itself. Some forms of overfishing, such as the overfishing of sharks, has led to the upset of entire marine ecosystems. Types of overfishing include growth overfishing, recruitment overfishing, and ecosystem overfishing. Overfishing not only causes negative impacts on biodiversity and ecosystem functioning, but also reduces fish production, which subsequently leads to negative social and economic consequences.

The fishing industry includes any industry or activity that takes, cultures, processes, preserves, stores, transports, markets or sells fish or fish products. It is defined by the Food and Agriculture Organization as including recreational, subsistence and commercial fishing, as well as the related harvesting, processing, and marketing sectors. The commercial activity is aimed at the delivery of fish and other seafood products for human consumption or as input factors in other industrial processes. The livelihood of over 500 million people in developing countries depends directly or indirectly on fisheries and aquaculture.

The Marine Stewardship Council (MSC) is a non-profit organisation which aims to set standards for sustainable fishing. Fisheries that wish to demonstrate they are well-managed and sustainable compared to the MSC's standards are assessed by a team of Conformity Assessment Bodies (CABs).
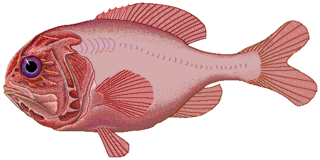
Slimeheads, also known as roughies and redfish, are mostly small, exceptionally long-lived, deep-sea beryciform fish constituting the family Trachichthyidae. Found in temperate to tropical waters of the Atlantic, Indian, and Pacific Oceans, the family comprises about 50 species in eight genera. Slimeheads are named for the network of muciferous canals riddling their heads.

Demersal fish, also known as groundfish, live and feed on or near the bottom of seas or lakes. They occupy the sea floors and lake beds, which usually consist of mud, sand, gravel or rocks. In coastal waters, they are found on or near the continental shelf, and in deep waters, they are found on or near the continental slope or along the continental rise. They are not generally found in the deepest waters, such as abyssal depths or on the abyssal plain, but they can be found around seamounts and islands. The word demersal comes from the Latin demergere, which means to sink.
The South Tasman Rise is an area of seafloor that lies 550 km south of Hobart, Tasmania in the Southern Ocean where water depths are about 1,500 metres. The South Tasman Rise is also known as the Tasmania Ridge or South Tasmania Ridge. The South Tasman Rise is a sunken landbridge that used to connect Tasmania to Antarctica.

The environmental impact of fishing includes issues such as the availability of fish, overfishing, fisheries, and fisheries management; as well as the impact of industrial fishing on other elements of the environment, such as bycatch. These issues are part of marine conservation, and are addressed in fisheries science programs. According to a 2019 FAO report, global production of fish, crustaceans, molluscs and other aquatic animals has continued to grow and reached 172.6 million tonnes in 2017, with an increase of 4.1 percent compared with 2016. There is a growing gap between the supply of fish and demand, due in part to world population growth.

The End of the Line: How Overfishing Is Changing the World and What We Eat is a book by journalist Charles Clover about overfishing. It was made into a movie released in 2009 and was re-released with updates in 2017.
The South East Atlantic Fisheries Organisation (SEAFO) is an organization that maintains controls over fishing and fishing related acts in the Southeastern Atlantic Ocean.

Sebastes levis, the cowcod or cow rockfish, is a species of marine ray-finned fish belonging to the subfamily Sebastinae, the rockfishes, part of the family Scorpaenidae. It is found in the eastern Pacific Ocean.

As with other countries, New Zealand's 200 nautical miles exclusive economic zone gives its fishing industry special fishing rights. It covers 4.1 million square kilometres. This is the sixth largest zone in the world, and is fourteen times the land area of New Zealand.

Friend of the Sea is a project of the World Sustainability Organization for the certification and promotion of seafood from sustainable fisheries and sustainable aquaculture. It is the only certification scheme which, with the same logo, certifies both wild and farmed seafood.

As with other countries, the 200 nautical miles (370 km) exclusive economic zone (EEZ) off the coast of the United States gives its fishing industry special fishing rights. It covers 11.4 million square kilometres, which is the second largest zone in the world, exceeding the land area of the United States.

Stock assessments provide fisheries managers with the information that is used in the regulation of a fish stock. Biological and fisheries data are collected in a stock assessment.

The Chilean jack mackerel, sometimes called the Jurel, Inca scad or Peruvian jack mackerel, is a species of jack mackerel in the genus Trachurus of the family Carangidae. Since the 1970s, it has become one of the world's more important commercial fish species. High volumes have been harvested, but the fishery may now be in danger of collapsing.

Hoplostethus is a genus of fish in the slimehead family.
Dianne Margaret Tracey is a New Zealand marine biologist specializing in research on deep-sea fisheries and deep-sea corals at the National Institute of Water and Atmospheric Research (NIWA). She works on the biology of deep water fishes such as orange roughy, and deep sea corals. She was one of the first women in New Zealand to work in fisheries and to work on research vessels and has spent her career advocating and mentoring women in marine science.

























