The actinide or actinoid series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Protactinium is a chemical element; it has symbol Pa and atomic number 91. It is a dense, radioactive, silvery-gray actinide metal which readily reacts with oxygen, water vapor, and inorganic acids. It forms various chemical compounds, in which protactinium is usually present in the oxidation state +5, but it can also assume +4 and even +3 or +2 states. Concentrations of protactinium in the Earth's crust are typically a few parts per trillion, but may reach up to a few parts per million in some uraninite ore deposits. Because of its scarcity, high radioactivity, and high toxicity, there are currently no uses for protactinium outside scientific research, and for this purpose, protactinium is mostly extracted from spent nuclear fuel.

Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive gray when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high melting point. Thorium is an electropositive actinide whose chemistry is dominated by the +4 oxidation state; it is quite reactive and can ignite in air when finely divided.

Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the cerium-dominant member of the group. It occurs usually in small isolated crystals. It has a hardness of 5.0 to 5.5 on the Mohs scale of mineral hardness and is relatively dense, about 4.6 to 5.7 g/cm3. There are five different most common species of monazite, depending on the relative amounts of the rare earth elements in the mineral:

Thorium dioxide (ThO2), also called thorium(IV) oxide, is a crystalline solid, often white or yellow in colour. Also known as thoria, it is produced mainly as a by-product of lanthanide and uranium production. Thorianite is the name of the mineralogical form of thorium dioxide. It is moderately rare and crystallizes in an isometric system. The melting point of thorium oxide is 3300 °C – the highest of all known oxides. Only a few elements (including tungsten and carbon) and a few compounds (including tantalum carbide) have higher melting points. All thorium compounds, including the dioxide, are radioactive because there are no stable isotopes of thorium.
Halocarbon compounds are chemicals in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms resulting in the formation of organofluorine compounds, organochlorine compounds, organobromine compounds, and organoiodine compounds. Chlorine halocarbons are the most common and are called organochlorides.
Uranium–thorium dating, also called thorium-230 dating, uranium-series disequilibrium dating or uranium-series dating, is a radiometric dating technique established in the 1960s which has been used since the 1970s to determine the age of calcium carbonate materials such as speleothem or coral. Unlike other commonly used radiometric dating techniques such as rubidium–strontium or uranium–lead dating, the uranium-thorium technique does not measure accumulation of a stable end-member decay product. Instead, it calculates an age from the degree to which secular equilibrium has been restored between the radioactive isotope thorium-230 and its radioactive parent uranium-234 within a sample.

The thorium fuel cycle is a nuclear fuel cycle that uses an isotope of thorium, 232
Th
, as the fertile material. In the reactor, 232
Th
is transmuted into the fissile artificial uranium isotope 233
U
which is the nuclear fuel. Unlike natural uranium, natural thorium contains only trace amounts of fissile material, which are insufficient to initiate a nuclear chain reaction. Additional fissile material or another neutron source is necessary to initiate the fuel cycle. In a thorium-fuelled reactor, 232
Th
absorbs neutrons to produce 233
U
. This parallels the process in uranium breeder reactors whereby fertile 238
U
absorbs neutrons to form fissile 239
Pu
. Depending on the design of the reactor and fuel cycle, the generated 233
U
either fissions in situ or is chemically separated from the used nuclear fuel and formed into new nuclear fuel.

Thorium(IV) chloride describes a family of inorganic compounds with the formula ThCl4(H2O)n. Both the anhydrous and tetrahydrate (n = 4) forms are known. They are hygroscopic, water-soluble white salts.

Thorium(IV) fluoride (ThF4) is an inorganic chemical compound. It is a white hygroscopic powder which can be produced by reacting thorium with fluorine gas. At temperatures above 500 °C, it reacts with atmospheric moisture to produce ThOF2.
Thorium(IV) orthosilicate (ThSiO4) is an inorganic chemical compound. Thorite is a mineral that consists essentially of thorium othosilicate.

Thorium(IV) sulfide (ThS2) is an inorganic chemical compound composed of one thorium atom ionically bonded to two atoms of sulfur. This salt is dark brown and has a melting point of 1905 °C. ThS2 adopts the same orthorhombic lattice structure as PbCl2.

Huttonite is a thorium nesosilicate mineral with the chemical formula ThSiO4 and which crystallizes in the monoclinic system. It is dimorphous with tetragonal thorite, and isostructual with monazite. An uncommon mineral, huttonite forms transparent or translucent cream–colored crystals. It was first identified in samples of beach sands from the West Coast region of New Zealand by the mineralogist Colin Osborne Hutton (1910–1971). Owing to its rarity, huttonite is not an industrially useful mineral.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.

Thorium(IV) nitrate is a chemical compound, a salt of thorium and nitric acid with the formula Th(NO3)4. A white solid in its anhydrous form, it can form tetra- and pentahydrates. As a salt of thorium it is weakly radioactive.
Thorium monoxide, is the binary oxide of thorium having chemical formula ThO. The covalent bond in this diatomic molecule is highly polar. The effective electric field between the two atoms has been calculated to be about 80 gigavolts per centimeter, one of the largest known internal effective electric fields.
Thorium(IV) hydroxide is an inorganic compound with a chemical formula Th(OH)4.
Protactinium compounds are compounds containing the element protactinium. These compounds usually have protactinium in the +5 oxidation state, although these compounds can also exist in the +2, +3 and +4 oxidation states.
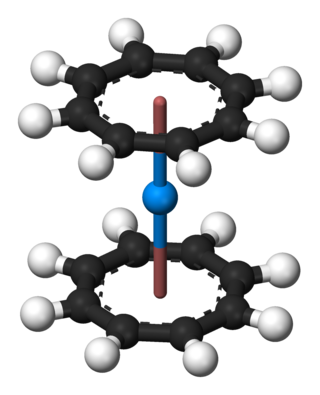
Organothorium chemistry describes the synthesis and properties of organothorium compounds, chemical compounds containing a carbon to thorium chemical bond.
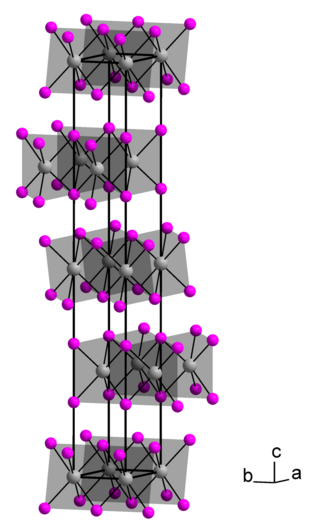
Thorium diiodide is an iodide of thorium, with the chemical formula of ThI2. It is an electride with the ionic formula Th4+(I-)2e-2. It is air-sensitive.











