Contents
- Etymology
- History
- Chemical properties
- Acidity
- Salts
- Esters
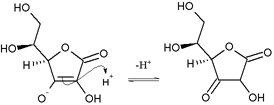
- Nucleophilic attack
- Oxidation
- Other reactions
- Uses
- Food additive
- Dietary supplement and biological relevance
- Niche, non-food uses
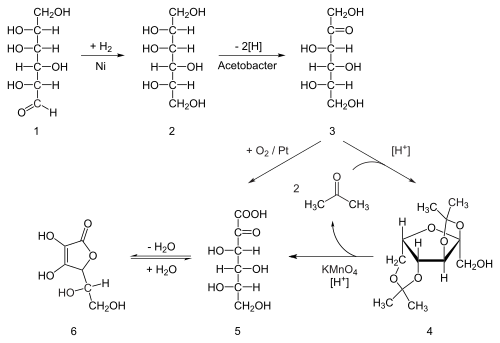
- Synthesis
- Industrial preparation
- Determination
- See also
- References
- Further reading
- External links
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name (5R)-[(1S)-1,2-Dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| E number | E300 (antioxidants, ...) | ||
| KEGG | |||
PubChem CID | |||
| UNII | |||
| |||
| |||
| Properties | |||
| C6H8O6 | |||
| Molar mass | 176.124 g·mol−1 | ||
| Appearance | White or light yellow solid | ||
| Density | 1.65 g/cm3 | ||
| Melting point | 190 to 192 °C (374 to 378 °F; 463 to 465 K) decomposes | ||
| 330 g/L | |||
| Solubility | Insoluble in diethyl ether, chloroform, benzene, petroleum ether, oils, fats | ||
| Solubility in ethanol | 20 g/L | ||
| Solubility in glycerol | 10 g/L | ||
| Solubility in propylene glycol | 50 g/L | ||
| Acidity (pKa) | 4.10 (first), 11.6 (second) | ||
| Pharmacology | |||
| A11GA01 ( WHO ) G01AD03 ( WHO ), S01XA15 ( WHO ) | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) | 11.9 g/kg (oral, rat) [1] | ||
| Safety data sheet (SDS) | JT Baker | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Ascorbic acid is an organic compound with formula C6H8O6, originally called hexuronic acid. It is a white solid, but impure samples can appear yellowish. It dissolves freely in water to give mildly acidic solutions. It is a mild reducing agent.
Ascorbic acid exists as two enantiomers (mirror-image isomers), commonly denoted "l" (for "levo") and "d" (for "dextro"). The l isomer is the one most often encountered: it occurs naturally in many foods, and is one form ("vitamer") of vitamin C, an essential nutrient for humans and many animals. [2] Deficiency of vitamin C causes scurvy, formerly a major disease of sailors in long sea voyages. [3] It is used as a food additive and a dietary supplement for its antioxidant properties. The "d" form (erythorbic acid) can be made by chemical synthesis, but has no significant biological role.