Related Research Articles

A nuclear pore is a part of a large complex of proteins, known as a nuclear pore complex that spans the nuclear envelope, which is the double membrane surrounding the eukaryotic cell nucleus. There are approximately 1,000 nuclear pore complexes (NPCs) in the nuclear envelope of a vertebrate cell, but this number varies depending on cell type and the stage in the life cycle. The human nuclear pore complex (hNPC) is a 110 megadalton (MDa) structure. The proteins that make up the nuclear pore complex are known as nucleoporins; each NPC contains at least 456 individual protein molecules and is composed of 34 distinct nucleoporin proteins. About half of the nucleoporins typically contain solenoid protein domains—either an alpha solenoid or a beta-propeller fold, or in some cases both as separate structural domains. The other half show structural characteristics typical of "natively unfolded" or intrinsically disordered proteins, i.e. they are highly flexible proteins that lack ordered tertiary structure. These disordered proteins are the FG nucleoporins, so called because their amino-acid sequence contains many phenylalanine–glycine repeats.
A nuclear localization signalorsequence (NLS) is an amino acid sequence that 'tags' a protein for import into the cell nucleus by nuclear transport. Typically, this signal consists of one or more short sequences of positively charged lysines or arginines exposed on the protein surface. Different nuclear localized proteins may share the same NLS. An NLS has the opposite function of a nuclear export signal (NES), which targets proteins out of the nucleus.
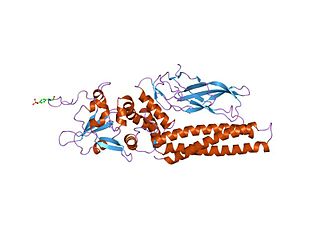
Members of the signal transducer and activator of transcription (STAT) protein family are intracellular transcription factors that mediate many aspects of cellular immunity, proliferation, apoptosis and differentiation. They are primarily activated by membrane receptor-associated Janus kinases (JAK). Dysregulation of this pathway is frequently observed in primary tumors and leads to increased angiogenesis which enhances the survival of tumors and immunosuppression. Gene knockout studies have provided evidence that STAT proteins are involved in the development and function of the immune system and play a role in maintaining immune tolerance and tumor surveillance.
Karyopherins are proteins involved in transporting molecules between the cytoplasm and the nucleus of a eukaryotic cell. The inside of the nucleus is called the karyoplasm. Generally, karyopherin-mediated transport occurs through nuclear pores which act as a gateway into and out of the nucleus. Most proteins require karyopherins to traverse the nuclear pore.
Importin is a type of karyopherin that transports protein molecules from the cell's cytoplasm to the nucleus. It does so by binding to specific recognition sequences, called nuclear localization sequences (NLS).
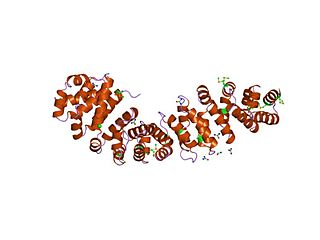
An armadillo repeat is the name of a characteristic, repetitive amino acid sequence of about 42 residues in length that is found in many proteins. Proteins that contain armadillo repeats typically contain several tandemly repeated copies. Each armadillo repeat is composed of a pair of alpha helices that form a hairpin structure. Multiple copies of the repeat form what is known as an alpha solenoid structure.

Ran also known as GTP-binding nuclear protein Ran is a protein that in humans is encoded by the RAN gene. Ran is a small 25 kDa protein that is involved in transport into and out of the cell nucleus during interphase and also involved in mitosis. It is a member of the Ras superfamily.
Nuclear transport refers to the mechanisms by which molecules move across the nuclear membrane of a cell. The entry and exit of large molecules from the cell nucleus is tightly controlled by the nuclear pore complexes (NPCs). Although small molecules can enter the nucleus without regulation, macromolecules such as RNA and proteins require association with transport factors known as nuclear transport receptors, like karyopherins called importins to enter the nucleus and exportins to exit.

Nucleoporins are a family of proteins which are the constituent building blocks of the nuclear pore complex (NPC). The nuclear pore complex is a massive structure embedded in the nuclear envelope at sites where the inner and outer nuclear membranes fuse, forming a gateway that regulates the flow of macromolecules between the cell nucleus and the cytoplasm. Nuclear pores enable the passive and facilitated transport of molecules across the nuclear envelope. Nucleoporins, a family of around 30 proteins, are the main components of the nuclear pore complex in eukaryotic cells. Nucleoporin 62 is the most abundant member of this family. Nucleoporins are able to transport molecules across the nuclear envelope at a very high rate. A single NPC is able to transport 60,000 protein molecules across the nuclear envelope every minute.

Importin subunit alpha-1 is a protein that in humans is encoded by the KPNA2 gene.

Importin subunit beta-1 is a protein that in humans is encoded by the KPNB1 gene.

Importin subunit alpha-5 is a protein that in humans is encoded by the KPNA1 gene.

Importin subunit alpha-4 also known as karyopherin subunit alpha-3 is a protein that in humans is encoded by the KPNA3 gene.

Importin subunit alpha-7 is a protein that in humans is encoded by the KPNA6 gene.
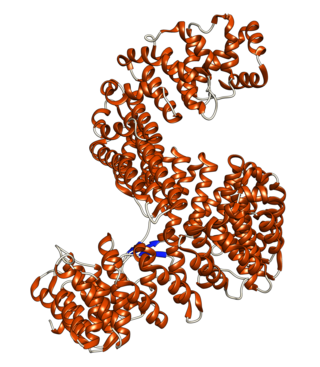
Importin-5 is a protein that in humans is encoded by the IPO5 gene. The protein encoded by this gene is a member of the importin beta family. Structurally, the protein adopts the shape of a right hand solenoid and is composed of 24 HEAT repeats.

Nucleoporin 153 (Nup153) is a protein which in humans is encoded by the NUP153 gene. It is an essential component of the basket of nuclear pore complexes (NPCs) in vertebrates, and required for the anchoring of NPCs. It also acts as the docking site of an importing karyopherin. On the cytoplasmic side of the NPC, Nup358 fulfills an analogous role.

Transportin-1 is a protein that in humans is encoded by the TNPO1 gene.

Importin-13 is a protein encoded by the IPO13 gene in humans. Importin-13 is a member of the importin-β family of nuclear transport receptors (NTRs) and was first identified as a transport receptor in 2000. According to PSI-blast based secondary structure PREDiction (PSIPRED), importin-13 contains 38 α-helices. Importin-13 accommodates a range of cargoes due to its flexible superhelical structure and a cargo binding and release system that is distinct from other importin-like transport receptors. IPO13 is broadly expressed in a variety of tissues in the human body, including the heart, cornea, fetal lung, brain, endometrial carcinoma, and testes.
A nuclear export signal (NES) is a short target peptide containing 4 hydrophobic residues in a protein that targets it for export from the cell nucleus to the cytoplasm through the nuclear pore complex using nuclear transport. It has the opposite effect of a nuclear localization signal, which targets a protein located in the cytoplasm for import to the nucleus. The NES is recognized and bound by exportins.

Rev is a transactivating protein that is essential to the regulation of HIV-1 protein expression. A nuclear localization signal is encoded in the rev gene, which allows the Rev protein to be localized to the nucleus, where it is involved in the export of unspliced and incompletely spliced mRNAs. In the absence of Rev, mRNAs of the HIV-1 late (structural) genes are retained in the nucleus, preventing their translation.
References
- ↑ Köhler, Matthias; Speck, Christian; Christiansen, Marret; Bischoff, F. Ralf; Prehn, Siegfried; Haller, Hermann; Görlich, Dirk; Hartmann, Enno (1 November 1999). "Evidence for Distinct Substrate Specificities of Importin α Family Members in Nuclear Protein Import". Molecular and Cellular Biology. 19 (11): 7782–7791. doi:10.1128/MCB.19.11.7782. ISSN 0270-7306. PMC 84838 . PMID 10523667.
- 1 2 Moroianu, J; Blobel, G; Radu, A (14 March 1995). "Previously identified protein of uncertain function is karyopherin alpha and together with karyopherin beta docks import substrate at nuclear pore complexes". Proceedings of the National Academy of Sciences of the United States of America. 92 (6): 2008–2011. Bibcode:1995PNAS...92.2008M. doi: 10.1073/pnas.92.6.2008 . ISSN 0027-8424. PMC 42412 . PMID 7892216.
- 1 2 3 4 5 6 7 8 Goldfarb, David S.; Corbett, Anita H.; Mason, D. Adam; Harreman, Michelle T.; Adam, Stephen A. (2004). "Importin α: a multipurpose nuclear-transport receptor". Trends in Cell Biology. 14 (9): 505–514. doi:10.1016/j.tcb.2004.07.016. PMID 15350979.
- 1 2 Görlich, D; Henklein, P; Laskey, R A; Hartmann, E (15 April 1996). "A 41 amino acid motif in importin-alpha confers binding to importin-beta and hence transit into the nucleus". The EMBO Journal. 15 (8): 1810–1817. doi:10.1002/j.1460-2075.1996.tb00530.x. ISSN 0261-4189. PMC 450097 . PMID 8617226.
- ↑ Lange, Allison; Mills, Ryan E.; Lange, Christopher J.; Stewart, Murray; Devine, Scott E.; Corbett, Anita H. (23 February 2007). "Classical Nuclear Localization Signals: Definition, Function, and Interaction with Importin α". Journal of Biological Chemistry. 282 (8): 5101–5105. doi: 10.1074/jbc.R600026200 . ISSN 0021-9258. PMC 4502416 . PMID 17170104.
- ↑ Kobe, Bostjan (1 April 1999). "Autoinhibition by an internal nuclear localization signal revealed by the crystal structure of mammalian importin α". Nature Structural & Molecular Biology. 6 (4): 388–397. doi:10.1038/7625. ISSN 1072-8368. PMID 10201409. S2CID 20026752.
- ↑ Moroianu, J; Blobel, G; Radu, A (25 June 1996). "The binding site of karyopherin alpha for karyopherin beta overlaps with a nuclear localization sequence". Proceedings of the National Academy of Sciences of the United States of America. 93 (13): 6572–6576. Bibcode:1996PNAS...93.6572M. doi: 10.1073/pnas.93.13.6572 . ISSN 0027-8424. PMC 39066 . PMID 8692858.
- 1 2 Giarrè, Marianna; Török, Istvan; Schmitt, Rolf; Gorjánácz, Mátyás; Kiss, István; Mechler, Bernard M. (1 October 2002). "Patterns of importin-α expression during Drosophila spermatogenesis". Journal of Structural Biology. 140 (1–3): 279–290. doi:10.1016/S1047-8477(02)00543-9. PMID 12490175.
- 1 2 Fang, Xiang-dong; Chen, Tianxin; Tran, Kim; Parker, Carl S. (1 September 2001). "Developmental regulation of the heat shock response by nuclear transport factor karyopherin-α3". Development. 128 (17): 3349–3358. doi: 10.1242/dev.128.17.3349 . ISSN 0950-1991. PMID 11546751.
- 1 2 Geles, K. G.; Adam, S. A. (15 May 2001). "Germline and developmental roles of the nuclear transport factor importin (α)3 in C. elegans". Development. 128 (10): 1817–1830. doi:10.1242/dev.128.10.1817. ISSN 0950-1991. PMID 11311162.
- 1 2 Tejomurtula, Jyothsna; Lee, Kyung-Bon; Tripurani, Swamy K.; Smith, George W.; Yao, Jianbo (1 August 2009). "Role of Importin Alpha8, a New Member of the Importin Alpha Family of Nuclear Transport Proteins, in Early Embryonic Development in Cattle". Biology of Reproduction. 81 (2): 333–342. doi: 10.1095/biolreprod.109.077396 . ISSN 0006-3363. PMID 19420384.
- 1 2 Yasuhara, Noriko; Yamagishi, Ryosuke; Arai, Yoshiyuki; Mehmood, Rashid; Kimoto, Chihiro; Fujita, Toshiharu; Touma, Kenichi; Kaneko, Azumi; Kamikawa, Yasunao (2013). "Importin Alpha Subtypes Determine Differential Transcription Factor Localization in Embryonic Stem Cells Maintenance". Developmental Cell. 26 (2): 123–135. doi: 10.1016/j.devcel.2013.06.022 . PMID 23906064.
- 1 2 3 Hogarth, Cathryn A.; Calanni, Sophina; Jans, David A.; Loveland, Kate L. (1 January 2006). "Importin α mRNAs have distinct expression profiles during spermatogenesis". Developmental Dynamics. 235 (1): 253–262. doi: 10.1002/dvdy.20569 . ISSN 1097-0177. PMID 16261624. S2CID 86703945.
- ↑ Bier, Carolin; Knauer, Shirley K.; Docter, Dominic; Schneider, Günter; Krämer, Oliver H.; Stauber, Roland H. (1 June 2011). "The Importin-Alpha/Nucleophosmin Switch Controls Taspase1 Protease Function". Traffic. 12 (6): 703–714. doi: 10.1111/j.1600-0854.2011.01191.x . ISSN 1600-0854. PMID 21418451. S2CID 205841729.
- 1 2 Wang, P.; Palese, P.; O'Neill, R. E. (1 March 1997). "The NPI-1/NPI-3 (karyopherin alpha) binding site on the influenza a virus nucleoprotein NP is a nonconventional nuclear localization signal". Journal of Virology. 71 (3): 1850–1856. doi:10.1128/JVI.71.3.1850-1856.1997. ISSN 0022-538X. PMC 191255 . PMID 9032315.
- 1 2 Singhal, Prabhat K.; Kumar, P. Rajendra; Rao, Malireddi R. K. Subba; Kyasani, Mahesh; Mahalingam, Sundarasamy (1 January 2006). "Simian Immunodeficiency Virus Vpx Is Imported into the Nucleus via Importin Alpha-Dependent and -Independent Pathways". Journal of Virology. 80 (1): 526–536. doi:10.1128/JVI.80.1.526-536.2006. ISSN 0022-538X. PMC 1317556 . PMID 16352576.